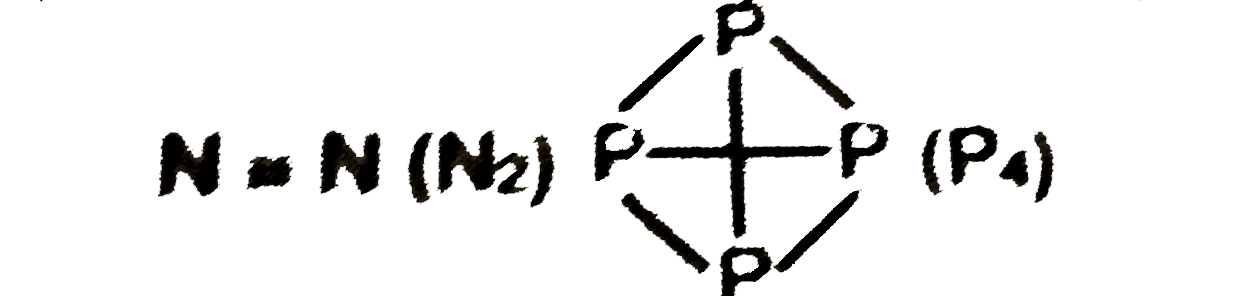Text Solution
Verified by Experts
Topper's Solved these Questions
NITROGEN & OXYGEN FAMILY
RESONANCE|Exercise Exercise 3 (Part-II: JEE MAIN)|13 VideosNITROGEN & OXYGEN FAMILY
RESONANCE|Exercise Exercise 3 (JEE (MAIN) Online problems)|11 VideosNITROGEN & OXYGEN FAMILY
RESONANCE|Exercise Exercise 2 (Part : IV Comprehension)|13 VideosMETALLURGY
RESONANCE|Exercise INORGANIC CHEMISTRY(Metallurgy)|42 VideosNITROGEN CONTAINING COMPOUNDS
RESONANCE|Exercise ORGANIC CHEMISTRY(Nitrogen containing Compounds)|30 Videos
Similar Questions
Explore conceptually related problems
RESONANCE-NITROGEN & OXYGEN FAMILY-Exercise 3 (Part-I: JEE Advanced)
- The correct order of acidic strength is
Text Solution
|
- Ammonia can be dried by :
Text Solution
|
- Give reason, why elemental nitrogen exists as a diatomic molecule, whe...
Text Solution
|
- Polyphosphates are used as water softening agents because they
Text Solution
|
- For H(3)PO(3) and H(3)PO(4) the correct choice is
Text Solution
|
- (NH(4))(2)Cr(2)O(7) on heating gives a gas which is also given by :
Text Solution
|
- A pale bule liquid is obtained by equuimolar mixture of two gases at -...
Text Solution
|
- Thermodynamically most stable allotrope of phosphorus is :
Text Solution
|
- (a) What amount of CaO in grams is required to neutralise 852g of P(4)...
Text Solution
|
- There are some deposits of nitrated and phosphates in the earth's crus...
Text Solution
|
- There are some deposits of nitrated and phosphates in the earth's crus...
Text Solution
|
- There are some deposits of nitrates and phosphates in the earth's crus...
Text Solution
|
- The reaction of P(4) with X leads selectively to P(4)O(6) The X is :
Text Solution
|
- Match each of the reaction given in Column I with the corresponding pr...
Text Solution
|
- Extra pure N2 can be obtained by heating
Text Solution
|
- Among the following, the number of compounds that can react with PCl(5...
Text Solution
|
- Which ordering of compounds is according to the decreasing order of th...
Text Solution
|
- Concentrated nitric acid upon long standing turns yellowish-brown due ...
Text Solution
|
- The pair(s) of reagents that yield paramagnetic species is/are
Text Solution
|
- The product formed in the reaction of SOCl2 (thionyl chloride) with wh...
Text Solution
|