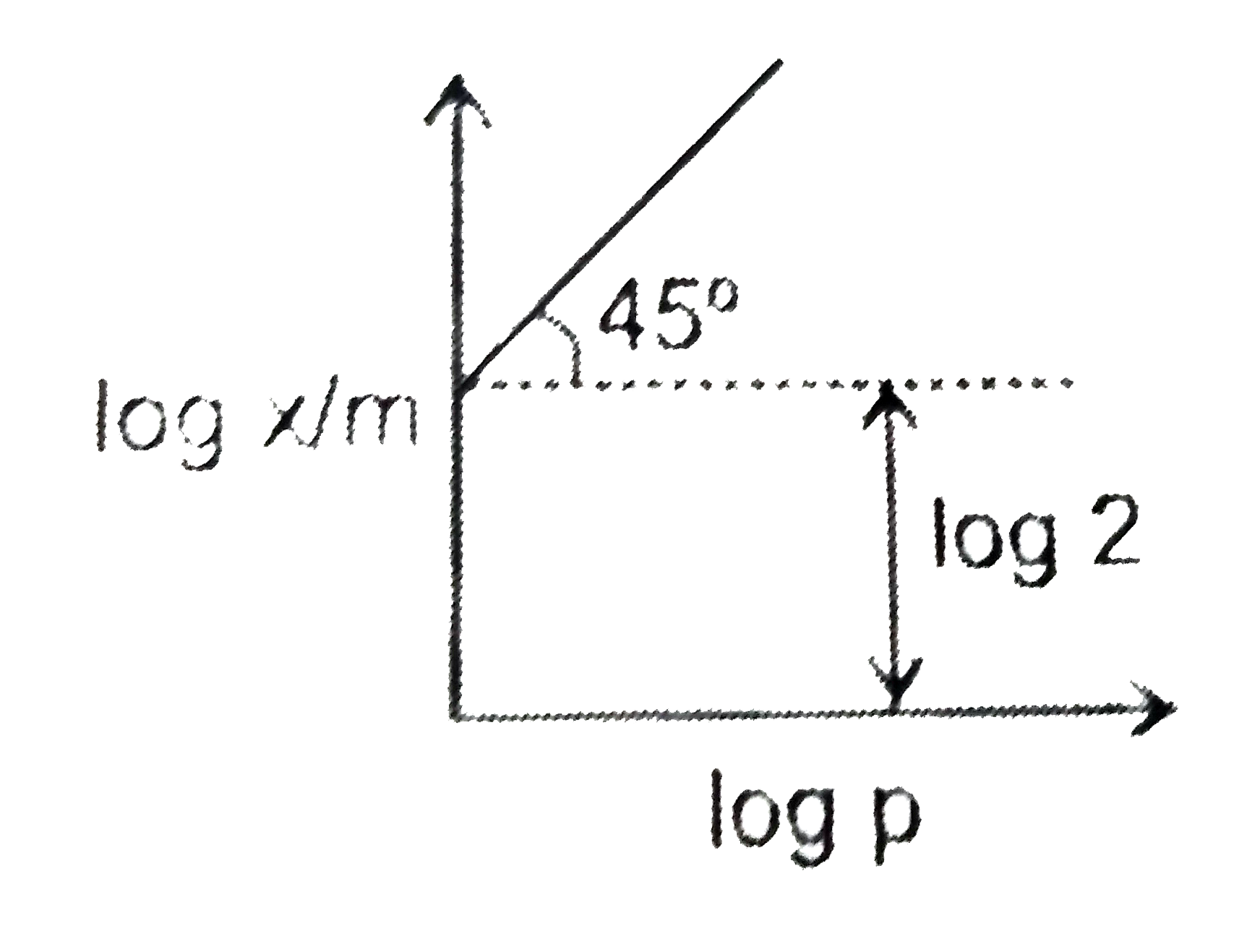
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SURFACE CHEMISTRY
RESONANCE|Exercise Section - 4|3 VideosSURFACE CHEMISTRY
RESONANCE|Exercise Section - 5|1 VideosSURFACE CHEMISTRY
RESONANCE|Exercise Section - 2|5 VideosSTRUCTURAL IDENTIFICATION
RESONANCE|Exercise Advanced level Problems (Part-III)|12 VideosTEST PAPERS
RESONANCE|Exercise PT-03|1 Videos
Similar Questions
Explore conceptually related problems
RESONANCE-SURFACE CHEMISTRY-Section - 3
- On addition of 1 mL solution of 10% NaCl to 10 mL gold sol in the pres...
Text Solution
|
- At 2 atm pressure the value of (x)/(m) will be : (log 2 = 0.3010)
Text Solution
|
- 1 L of 0.6 M acetic acid is shaken with 2 g activated carbon. Activate...
Text Solution
|
- A detergent (C(12)H(25)SO(4)Na) solution becomes a colloidal solution ...
Text Solution
|
- For the coagulation of 200 mL of As(2)S(3) solution, 10 mL of 1 M NaCl...
Text Solution
|
- A solution of palmitic acid (Molar mass = 256) in Benzene contain 5.12...
Text Solution
|