A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN-REDOX REACTIONS-Exercise - 1
- Reaction (A) S^(-2)+4H(2)O(2)rarrSO(4)^(2-)+4H(2)O (B) Cl(2)+H(2)O(2...
Text Solution
|
- A compound contains atoms A, B and C. The oxidation number A is +2, of...
Text Solution
|
- Equivalent weight of N(2) in the change N(2) rarr NH(3) is
Text Solution
|
- Equivalent weight of NH(3) in the change N(2)rarrNH(3) is :
Text Solution
|
- In the reaction, 2S(2)O(3)^(2-)+I(2)rarrS(4)O(6)^(2-)+2I^(-). The eq. ...
Text Solution
|
- In the reaction VO+Fe(2)O(3) rarr FeO+V(2)O(5), the eq.wt. of V(2)O(5)...
Text Solution
|
- In the reaction, I(2)+2S(2)O(3)^(2-) rarr 2I^(-)+S(4)O(6)^(2-). Eq...
Text Solution
|
- Molecular weight of KBrO(3) is M. What is its equivalent weight, if th...
Text Solution
|
- In the reaction A^(-n2)+xe^(-)rarrA^(-n1) . Here, x will be :
Text Solution
|
- What would be the equivalent weight of the reductant in the reaction :...
Text Solution
|
- The eq. wt. of Na(2)S(2)O(3) as reductant in the reaction, Na(2)S(2)...
Text Solution
|
- The equivalent weight of FeC(2)O(4) in the change FeC(2)O(4)rarrFe^(...
Text Solution
|
- What will be n-factor for Ba(MnO(4))(2) in acidic medium? (Where it be...
Text Solution
|
- The number of mole of oxalate ions oxidised by one mole of MnO(4)^(-) ...
Text Solution
|
- Oxidising product of substance Na(3)AsO(3) would be
Text Solution
|
- In a reaction, 4 mole of electrons are transferred to 1 mole of HNO(3)...
Text Solution
|
- Balance given following half reaction for the unbalanced whole reactio...
Text Solution
|
- Choose the set coefficients that correctly balances the following equa...
Text Solution
|
- In the reaction : MnO(4)^(-)+xH^(+)+n e^(-)rarrMn^(2+)+yH(2)O What is ...
Text Solution
|
- The number of electrons required to balanced the following equation- ...
Text Solution
|
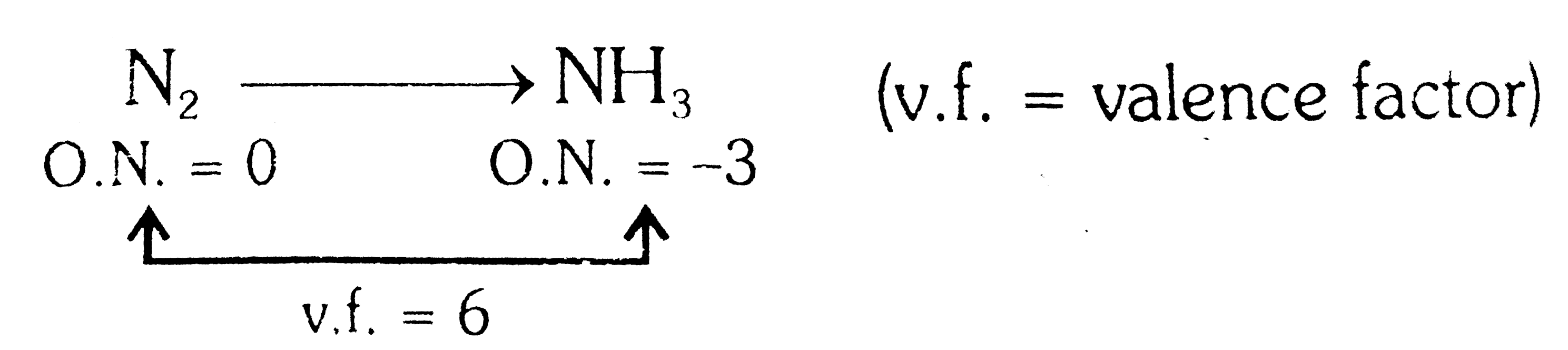 (v.f. = valence factor)
(v.f. = valence factor)