Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN-THERMODYNAMICS -EXERCISE -4
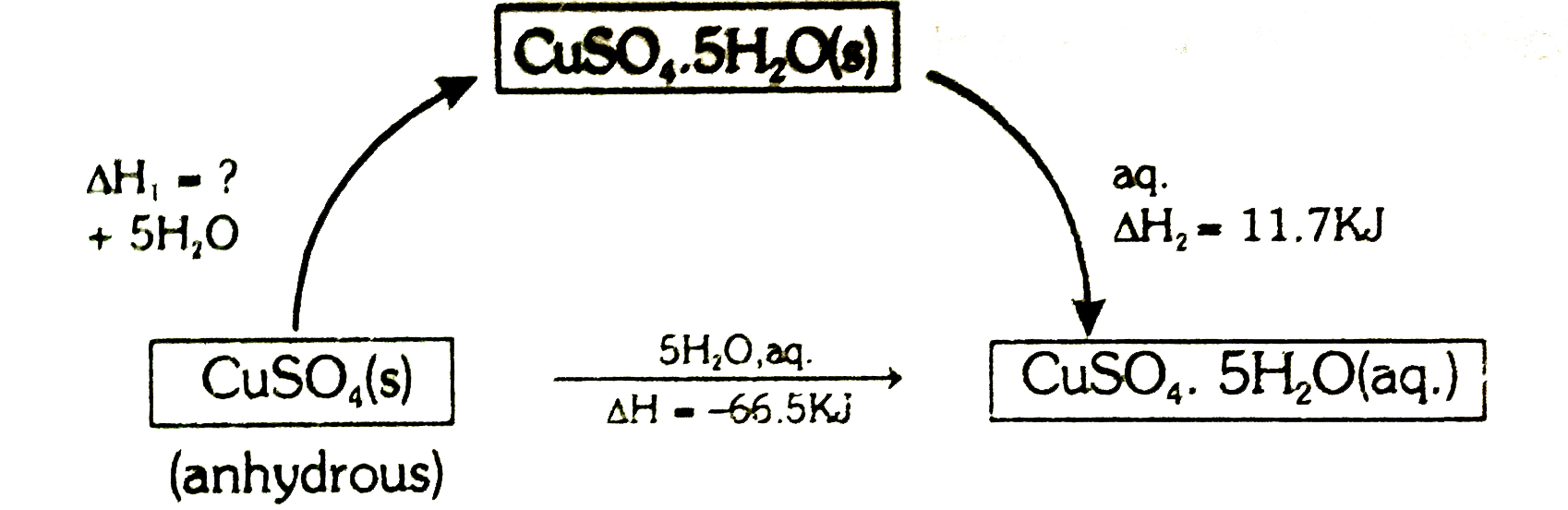
- The heat of solution of anhydrous CuSO(4) is -66.5 kJ and that of CuSO...
Text Solution
|
- Assertion (A): There is no reaction known for which DeltaG is positive...
Text Solution
|
- Assertion :- Absolute value of enthalpy can not be determined. Reaso...
Text Solution
|
- Assertion :- When a rubber band is stretched entropy increases. Reas...
Text Solution
|
- Assertion :- At constant pressure for the change H(2)O(s)rarr H(2)O(g)...
Text Solution
|
- Assertion (A): Enthalpy of graphite is lower than that of diamond. R...
Text Solution
|
- Assertion: The enthalpy of formation of gaseous oxygen molecules at 29...
Text Solution
|
- Assertion (A): May endothermic reactions that are not spontaneous at ...
Text Solution
|
- Assertion (A): Pressure, volume, and temperature are all extensive pro...
Text Solution
|
- Assertion (A): For a particular reaction, heat of combustion at consta...
Text Solution
|
- Assertion :- At constant temp 0^(@)C and 1 atm, the change H(2)O(s)rar...
Text Solution
|
- Assertion: The increase in internal energy (DeltaE) for the vaporisati...
Text Solution
|
- Assertion :- Water in liquid state is more stable than ice at room tem...
Text Solution
|
- Assertion :- In an isolated system the entropy increases due to sponta...
Text Solution
|
- Assertion :- Entropy is always constant for a closed system. Reason ...
Text Solution
|
- Assertion :- For an isolated system q is zero. Reason :- In an isola...
Text Solution
|
- Assertion :- Entropy of system increases for a spontaneous reactions. ...
Text Solution
|
- Assertion :- Catalyst change Gibbs free energy of system. Reason :- ...
Text Solution
|
- Assertion :- Q("Surr") is zero for adiabatic process Reason :- Final...
Text Solution
|