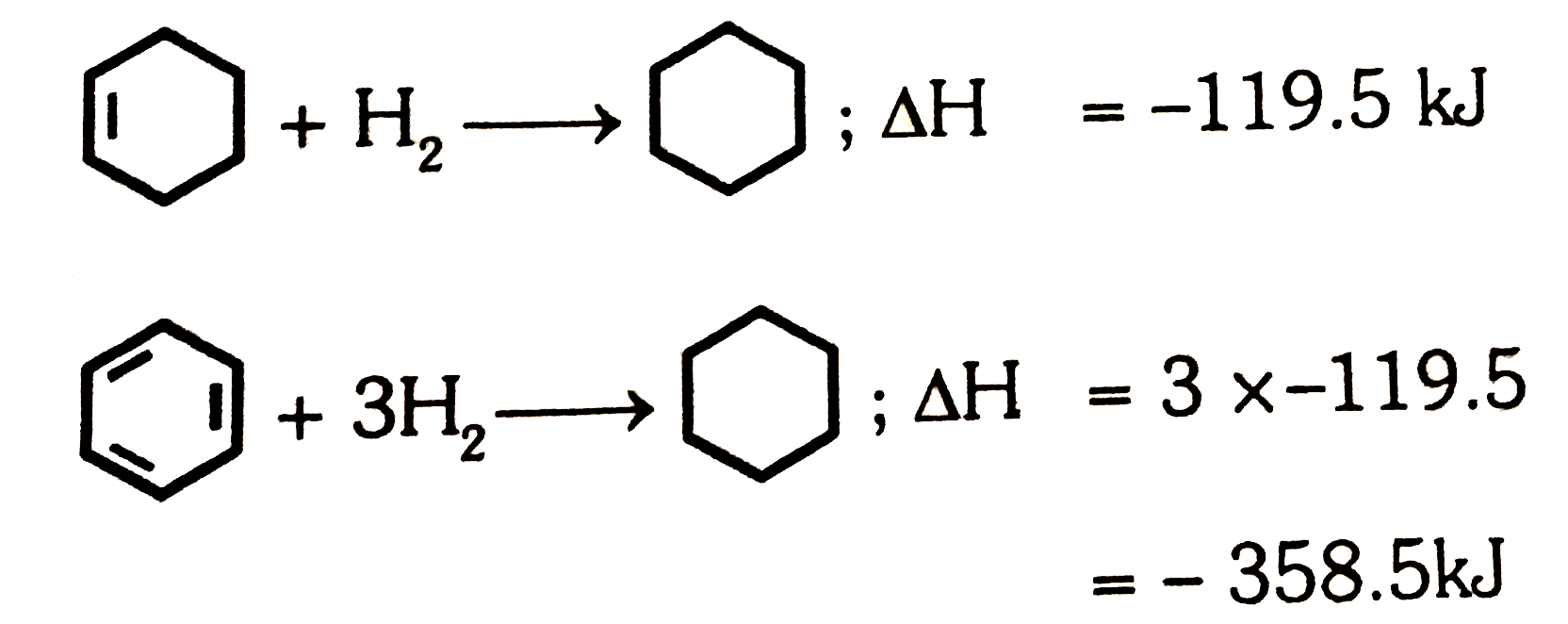A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN-THERMODYNAMICS -EXERCISE -1A
- Assume each reaction is carried out in an open container. For which ...
Text Solution
|
- The enthalpy and entropy change for the reaction, Br(2)(l)+Cl(2)(g)r...
Text Solution
|
- The enthalpy of hydrogenation of cyclohexene is -119.5kJ mol^(-1). If ...
Text Solution
|
- For the reaction of one mole of zinc dust with one mole of sulphuric a...
Text Solution
|
- When you make ice cubes, the entropy of water
Text Solution
|
- For a phase change: H(2)O(l)hArrH(2)O(s) 0^(@)C, 1 bar
Text Solution
|
- For a spontaneous process the correct statement is -
Text Solution
|
- The enthalpy change (Delta H) for the reaction N(2) (g)+3H(2)(g) rar...
Text Solution
|
- Consider the following reactions : (a) H((aq))^(+)+OH((aq))^(-)=H(2...
Text Solution
|
- Given the bond energies of H - H and Cl - Cl are 430 kJ mol^(-1) and 2...
Text Solution
|
- Bond dissociation enthalpy of H(2) , Cl(2) and HCl are 434, 242 and 43...
Text Solution
|
- For the gas phase reaction PCl(5)rarrPCl(3)(g)+Cl(2)(g) which of t...
Text Solution
|
- Which of the following are not state functions? (I) q+w (II)q (I...
Text Solution
|
- From the following bond energies: H--H bond energy: 431.37KJmol^(-1) ...
Text Solution
|
- The values of Delta H and Delta S for the reaction are 170 kJ and 17...
Text Solution
|
- For vaporization of water at 1 atmospheric pressure the values of Delt...
Text Solution
|
- Three moles of an ideal gas expanded spontaneously into vacuum. The wo...
Text Solution
|
- The following teo reaction are known : Fe(2)O(3)(s)+3CO(g)rarr2Fe(s)...
Text Solution
|
- Standard entropy of X(2) , Y(2) and XY(3) are 60, 40 and 50JK^(-1)mol...
Text Solution
|
- Intensive property is :-
Text Solution
|