Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-ATOMS -Chapter Practice Test
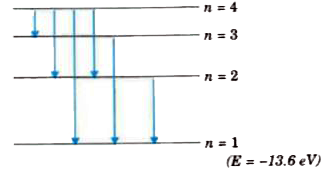
- Energy level diagram of a hydrogen atom is shown below : (i)If a ...
Text Solution
|
- An alpha-particle with kinetic energy K is heading towards a stationar...
Text Solution
|
- In which region of electromagnetic spectrum, Lyman and Balmer series o...
Text Solution
|
- The short wavelength limits of Lyman, Paschen and Balmer series in the...
Text Solution
|
- The innermost orbits of a hydrogen atom is 5.3xx10^(-11) m. What is th...
Text Solution
|
- With increasing quantum numbers, the energy difference between adjacen...
Text Solution
|
- In hydrogen spectrum, the shortest wavelength in Balmer series is the ...
Text Solution
|
- Draw the energy level diagram of hydrogen atom. Calculate the energy v...
Text Solution
|
- The electron in hydrogen atom passes form the n=4 energy level to the...
Text Solution
|
- for given impact parameter b, does the angle of deflection increase or...
Text Solution
|
- What is the shortest wavelength present in the Paschen series of spect...
Text Solution
|
- Using the Rydberg formula, calculate the wavelength of the first four ...
Text Solution
|
- Using Rutherford model of atom, derive an expression for the total ene...
Text Solution
|
- Calculate the velocity of electron in Bohr's first orbit of hydrogen a...
Text Solution
|
- The energy levels of an atom are as shown in figure . Which one of tho...
Text Solution
|
- The radius of the hydrogen atom in its ground state is 5.3xx10^(-11) m...
Text Solution
|
- Describe the Rutherford's alpha particle scattering experiment. What a...
Text Solution
|