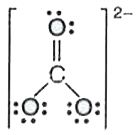
Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
MODERN PUBLICATION|Exercise Practice Problems|29 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
MODERN PUBLICATION|Exercise Conceptual Questions (1)|12 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
MODERN PUBLICATION|Exercise UNIT PRACTICE TEST|13 Videos
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-CHEMICAL BONDING AND MOLECULAR STRUCTURE-UNIT PRACTICE TEST
- Calculate the formal charge on each atoms in nitrite ion .
Text Solution
|
- Assertion : Bond length of O(2) is more than that of O(2)^(+). Reaso...
Text Solution
|
- The bond angle in H(2)O molecule is less than that of NH(3) molecule b...
Text Solution
|
- Which one of the following pairs of species have the same bond order ?
Text Solution
|
- The hybridization of atomic orbitals of nitrogen is NO(2)^(+), NO(3)^(...
Text Solution
|
- The incorrectly matched pair among the following is : {:("Molecule",...
Text Solution
|
- Is there any change in hybridisation of the B and N atom as a result o...
Text Solution
|
- Which of the two will have dipole moment? "cis or trans "-C(2)H(2)Cl...
Text Solution
|
- Which has higher dipole moment & why: NH(3),NF(3) ?
Text Solution
|
- Do ortho- nitrophenol and para - nitrophenol have hydrogen bonding in ...
Text Solution
|
- Giving one example explain the shapes of following molecules : (i) M...
Text Solution
|
- Explain the following : (i) Ionic compounds have high melting and bo...
Text Solution
|
- Discuss the shapes of following molecules using VSEPR model : (i) Si...
Text Solution
|
- (a) What is resonance ? Write resonance structures of carbon doxide mo...
Text Solution
|