Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
MODERN PUBLICATION|Exercise UNIT PRACTICE TEST|13 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
MODERN PUBLICATION|Exercise OBJECTIVE TYPE QUESTIONS (D. MULTIPLE CHOICE QUESTIONS)(Matrix Match Type Question)|6 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
MODERN PUBLICATION|Exercise UNIT PRACTICE TEST|13 Videos
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-CHEMICAL BONDING AND MOLECULAR STRUCTURE-OBJECTIVE TYPE QUESTIONS (D. MULTIPLE CHOICE QUESTIONS)(Integer Type Questions)
- The number of molecules or ions having bond order 2.5 among O(2)^(+), ...
Text Solution
|
- Total number of molecular orbitals occupying one or two electrons in O...
Text Solution
|
- Total number of coordinate bonds present in CuSO(4).5H(2)O is
Text Solution
|
- Total number of electrons present in piMOs in B(2) molecule is
Text Solution
|
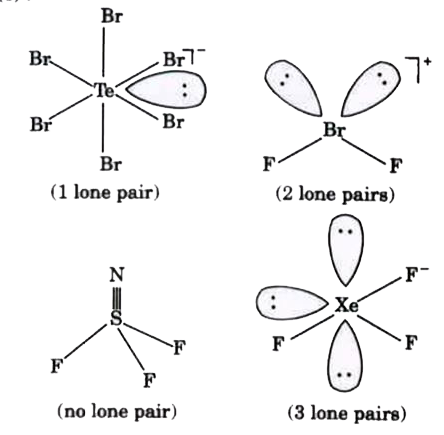
- The number of molecules having more than one lone pair among the follo...
Text Solution
|
- Number of molecules having dipole moment among : BF(3), H(2)O, NH(3), ...
Text Solution
|
- Find the total number of polar molecules SF(4),PCl(5),PCl(3)F(2),SF(...
Text Solution
|
- COCl(2) is a poisonous gas. The formal charge on O atom is
Text Solution
|
- The number of 90^(@) bond angles present in SF(4) molecules is
Text Solution
|
- Total number of lone pairs present in the structure of HNO(3) is
Text Solution
|
- Based on VSEPR theory, the number of 90 degree F-Br-F angles in BrF(5)...
Text Solution
|
- A list of species having the formula of XZ(4) is given below XeF(4), S...
Text Solution
|
- Among the triatomic molecules/ions BeCl(2),N(3)^(-),N(2)O, NO(2)^(+), ...
Text Solution
|
- Among H(2), He(2)^(+), Li(2), Be(2), B(2), C(2), N(2), O(2)^(-) and F(...
Text Solution
|
- The sum of the number of lone pair of electrons on each central atom i...
Text Solution
|