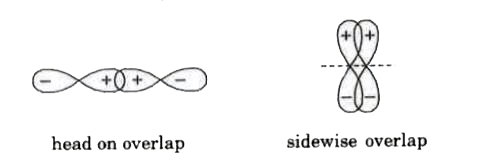
Molecular orbitals are formed by the overlap of atomic orbitals. The combining orbitals must have proper orientation so that they can overlap to a considerable extent. Two atomic orbitals combine to form two molecular orbitals called bonding molecular orbital and antibonding molecular orbital. Bonding molecular orbital is stable and antibonding molecular orbital is unstable. `sigma ` MO is formed by head on overlap while `pi` MO is formed by sidewise overlap.
The MOs are filled with electrons according to the same rules as followed for filling atomic orbitats. Bond order is one of the most important parameter to compare the bond strength and bond length of bonds.
Why has `N_2` a larger dissociation energy than `N_2 ^(+)` whereas `O_2` has a lower dissociation energy than `O_(2)^(+)`?