A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
MODERN PUBLICATION|Exercise Multiple Choice Questions (Level - III)|9 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
MODERN PUBLICATION|Exercise Recent Examination Questions|17 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
MODERN PUBLICATION|Exercise Recent Examination Questions|17 VideosBIOMOLECULES
MODERN PUBLICATION|Exercise Recent Examination Questions|13 VideosCHEMICAL KINETICS
MODERN PUBLICATION|Exercise Recent Examination Questions|20 Videos
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-CHEMICAL BONDING AND MOLECULAR STRUCTURE -Multiple Choice Questions (Level - II)
- In which of the following pairs, the two species are isostructural :
Text Solution
|
- Two types of FXF angles are present in which of the following molecule...
Text Solution
|
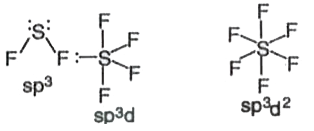
- SF(2), SF(4) and SF(6) have hybridisations at sulphur atom respective...
Text Solution
|
- Among the following, the compound that contains ionic, covalent and co...
Text Solution
|
- In which of the following species, all the three types of hybrid carbo...
Text Solution
|
- Which of the following represents the arrangement in increasing order ...
Text Solution
|
- In which one of the following species the central atom has the type of...
Text Solution
|
- Which one of the following pairs is isostructural (i.e., having the sa...
Text Solution
|
- The sp^(3)d^(2) hybridization of central atom of molecule would lead t...
Text Solution
|
- Which of the following are isoelectronic molecules ?
Text Solution
|
- When O(2) is converted to O(2)^(2-),
Text Solution
|
- Which one of the following is the correct statement ?
Text Solution
|
- In which of the following species is the underlined carbon having sp^(...
Text Solution
|
- Which of the following pair of molecules will have permanent dipole mo...
Text Solution
|
- Which of the following are isoelectronic and iso structural ? NO(3...
Text Solution
|
- The correct order of bond angle (smallest first) in H(2)S, NH(3), BF(3...
Text Solution
|
- The bond order in NO is 2.5 while that in NO^(+) is 3. Which of the fo...
Text Solution
|
- Which one of the following has the regular tetrahedral structure ?
Text Solution
|
- The maximum number of 90^(@) angles between bond pair - bond pair of e...
Text Solution
|
- According to MO theory, which of the following statements about the ma...
Text Solution
|