Similar Questions
Explore conceptually related problems
Recommended Questions
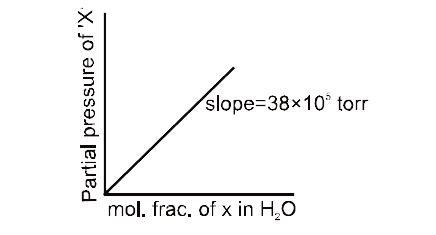
- A gas 'X' is present with saturated water vapour over water liquid at ...
Text Solution
|
- Two solutions of A and B are available. The first is known to contain ...
Text Solution
|
- An ideal mixture of liquids A and B with 2 moles of A and 2 moles of B...
Text Solution
|
- At room temperature the mole fraction of a solution is 0.25 and the va...
Text Solution
|
- A gas 'X' is present with saturated water vapour over water liquid at ...
Text Solution
|
- The vapour pressure of pure liquid A is 0.80 atm. On mixing a non-vola...
Text Solution
|
- A liquid is immiscible in water was steam distilled at 95.2^(@)C at a ...
Text Solution
|
- If the concentration of water vapour in the air is 1% and the total at...
Text Solution
|
- (i) 0.24 g of a gas dissolves in 1 L of water at 1.5 atm pressure. Cal...
Text Solution
|