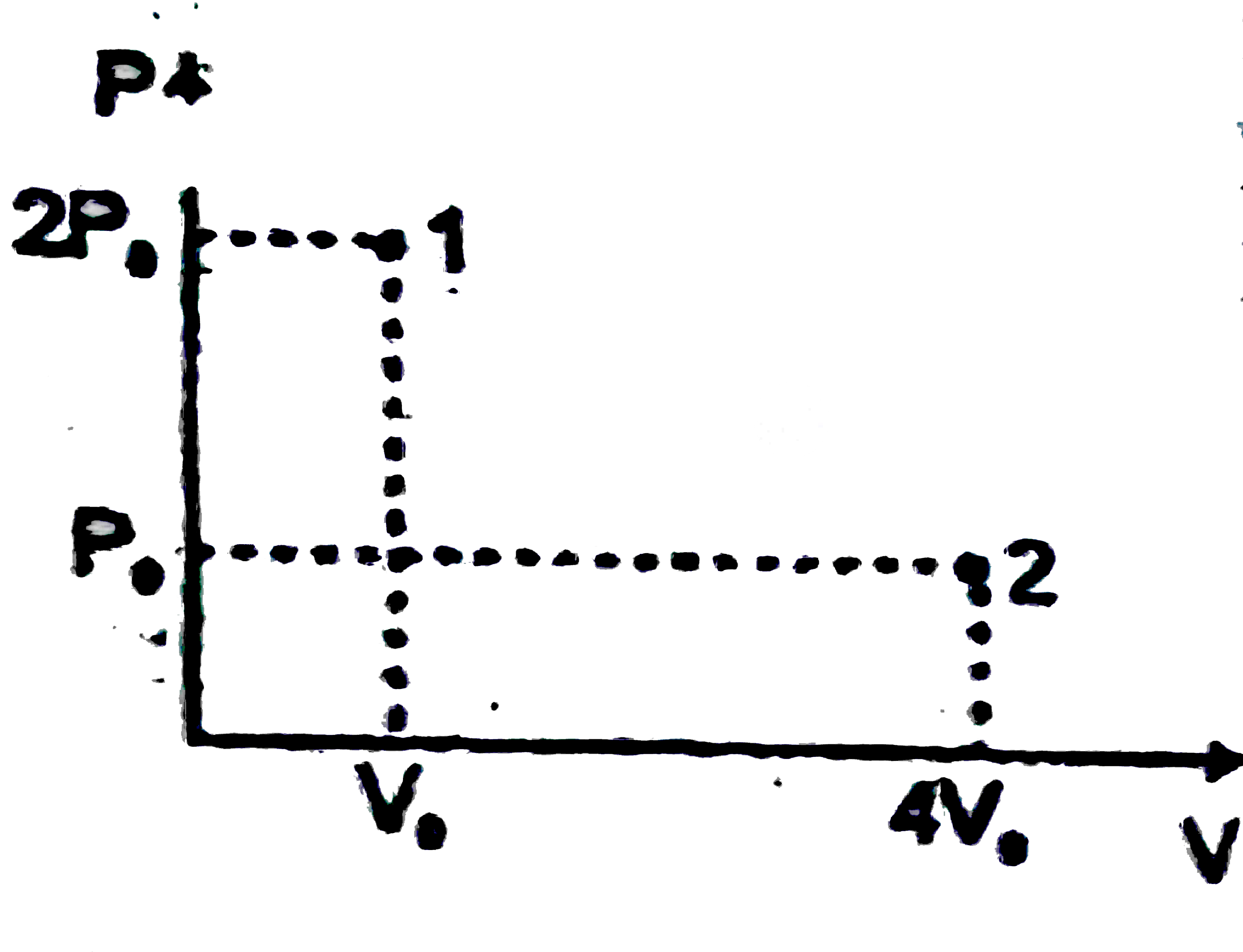
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- A liquid which is confined inside an adiabatic piston is suddently tak...
Text Solution
|
- A liquid which is confined inside an adiabatic piston is suddently tak...
Text Solution
|
- A liquid which is confined inside an adiabatic piston is suddenly take...
Text Solution
|
- A liquid confined inside an adiabatic container is suddenly taken fro...
Text Solution
|
- Which of the following are correct chain isomers of butane ? (i) <img ...
Text Solution
|
- Determine the point of symmetry of a regular hexagon. <img src="htt...
Text Solution
|
- Match the following Column A to Column B
Text Solution
|
- Match the following Column A to Column B
Text Solution
|
- Match the following Column A to Column B
Text Solution
|