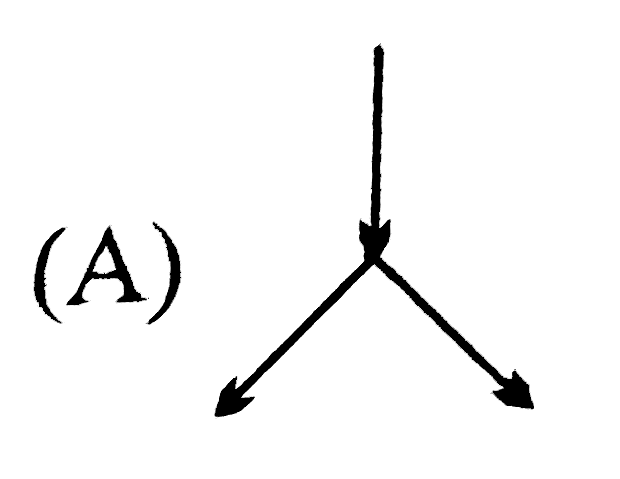
A
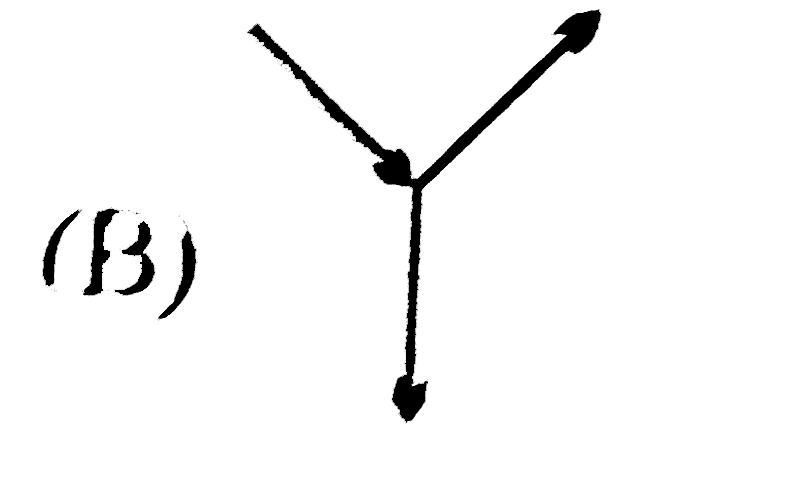
B
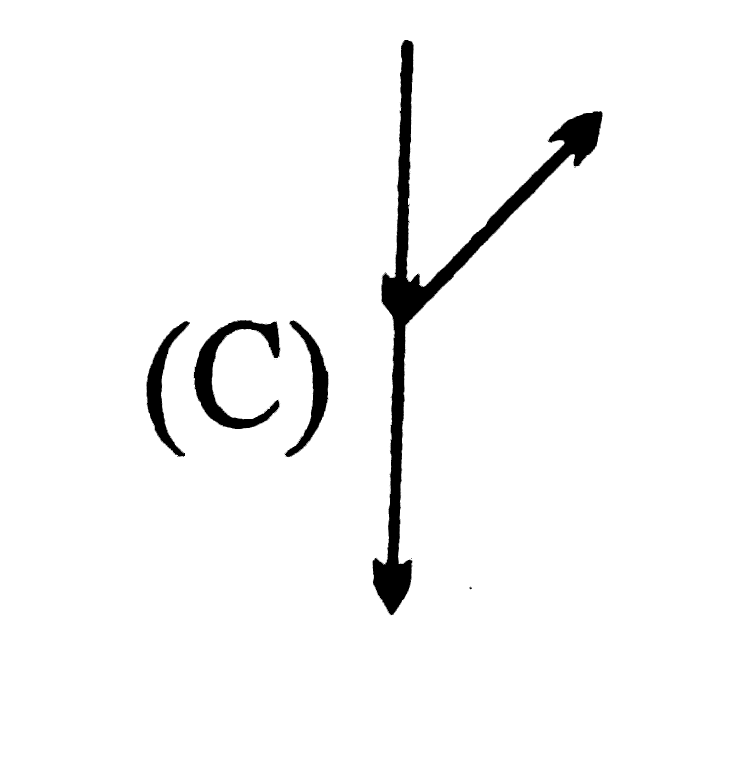
C
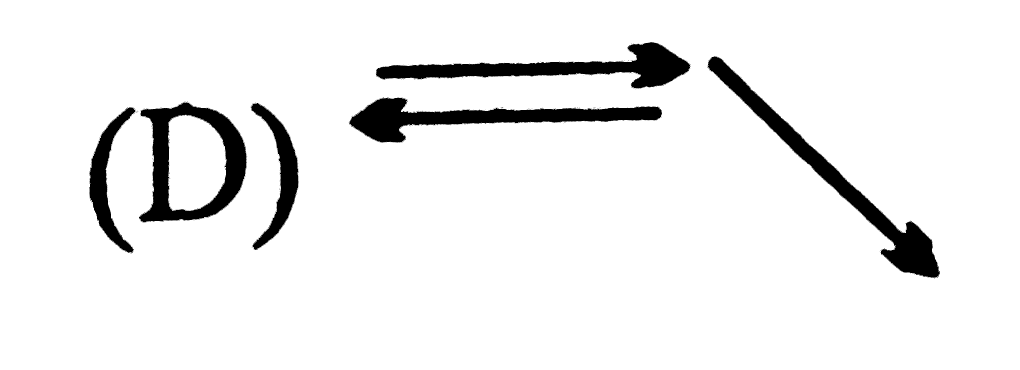
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BANSAL-TEST PAPERS-PHYSICS
- A Bohr hydrogen atom undergoes a transition n=5rarrn=4 and emits a pho...
Text Solution
|
- A stream of photons with energy 4.4eV strike a metal surface with work...
Text Solution
|
- When the voltage applied to an X-ray tube increased from V(1)15.5 KV...
Text Solution
|
- When a certain metallic surface is irradiated with monochromatic lig...
Text Solution
|
- Figure shows the graph of photocurrent versus potential of the anod...
Text Solution
|
- The energy level diagram of a hypothetical atom is shown in the figu...
Text Solution
|
- Suppose frequency of emitted photon is of when electron of a stationar...
Text Solution
|
- A cloud chamber is a device, which makes the tracks of moving charged ...
Text Solution
|
- The nuclear binding energies of the elements P and Q are E(P) and E(Q...
Text Solution
|
- A radioactive substance with decay constant of 0.5s^(-1) is being prod...
Text Solution
|
- Rydberg atoms are nearly classical atoms in which electron is excited ...
Text Solution
|
- Rydberg atoms are nearly classical atoms in which electron is excited ...
Text Solution
|
- The first nuclear reaction ever observed was by ernest Rutherford in 1...
Text Solution
|
- The first nuclear reaction ever observed was by ernest Rutherford in 1...
Text Solution
|
- In an electron transition inside a hydrogen atom, orbital angular mome...
Text Solution
|
- Which of the following are not dependent on the intensity of the inci...
Text Solution
|
- Consider a beam of light of power 7.2 W incident on a prism of angle ...
Text Solution
|
- An electron orbiting around the nucleus of an atom
Text Solution
|
- Shows the energy-level diagram of hydrogen like imaginary element X. (...
Text Solution
|
- The .^(238)U nucleus has a binding energy of about 7.6 MeV per nucleon...
Text Solution
|