Similar Questions
Explore conceptually related problems
Recommended Questions
- An engine operates by taking a monatomic ideal gas through the cycle ...
Text Solution
|
- The above p-v diagram represents the thermodymic cycle of an engine, o...
Text Solution
|
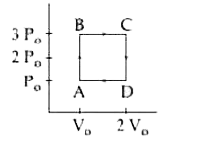
- Two moles of a monatomic ideal gas undergo a cyclic process ABCDA as s...
Text Solution
|
- A cycle followed an engine (made of one mole of an ideal gas in a cyl...
Text Solution
|
- An engine operates by taking n moles of an ideal gas through the cycle...
Text Solution
|
- Calculate the maximum % efficiency of thermal engine operating between...
Text Solution
|
- जल का गलनांक और क्वथनांक के बीच कार्यरत किसी आदर्श इंजन की दक्षता है...
Text Solution
|
- A perfect carnot engine utilizes an ideal gas the source temperature i...
Text Solution
|
- एक आदर्श कार्नो इंजन एक आदर्श गैस का उपयोग करता है। स्त्रोत का ताप 500...
Text Solution
|