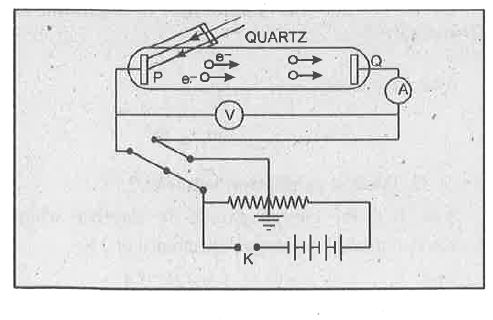
Text Solution
Verified by Experts
Topper's Solved these Questions
DUAL NATURE OF MATTER AND RADIATIONS
MBD -HARYANA BOARD|Exercise LONG ANSWER TYPE QUESTION|1 VideosDUAL NATURE OF MATTER AND RADIATIONS
MBD -HARYANA BOARD|Exercise OBJECTIVE TYPE QUESTIONS|17 VideosDUAL NATURE OF MATTER AND RADIATIONS
MBD -HARYANA BOARD|Exercise OBJECTIVE TYPE QUESTIONS|17 VideosCURRENT ELECTRICITY
MBD -HARYANA BOARD|Exercise OBJECTIVE TYPE QUESTIONS|20 VideosE.M.I. and A.C.
MBD -HARYANA BOARD|Exercise Exercise|10 Videos
Similar Questions
Explore conceptually related problems
MBD -HARYANA BOARD-DUAL NATURE OF MATTER AND RADIATIONS-SHORT ANSWER TYPE QUESTIONS
- If the frequency of incident radiation on a photocell is doubled for t...
Text Solution
|
- Describe photoelectric effect and state the laws of photoelectric emis...
Text Solution
|
- Explain photoelectric effect Explain the effect of increase of intensi...
Text Solution
|
- Define photoelectric work function. How is it related to thresold freq...
Text Solution
|
- Define photoelectric effect. Draw a graph to show the variation of sto...
Text Solution
|
- Explain effect of potential on photoelectric current.
Text Solution
|
- Draw a graph, showing the effect of potential on photoelectric current...
Text Solution
|
- Discuss the variation of photocurrent with collector plate potential f...
Text Solution
|
- What is photoelectric effect ? Explain effect of intensity on photoele...
Text Solution
|
- State Einstein’s photoelectric equation and explain the terms involved...
Text Solution
|
- Explain Einstein’s equation for photoelectric emission.
Text Solution
|
- Write Einstein’s photoelectric equation. Define the threshold frequenc...
Text Solution
|
- Describe photoelectric effect and state the laws of photoelectric emis...
Text Solution
|
- Define photoelectric effect and state the laws of photoelectric emissi...
Text Solution
|
- State two laws of photoelectric emission. Are cathode rays waves or pa...
Text Solution
|
- Explain the laws of photoelectric effect from the Einstein photoelectr...
Text Solution
|
- State Einstein's photoelectric equation. Explain any two characteris...
Text Solution
|
- State laws of photoelectric emission.
Text Solution
|
- Show that the de-Broglie wavelength A of an electron of energy E is gi...
Text Solution
|
- Derive expression for the de-Broglie wavelength of an electron moving ...
Text Solution
|