Text Solution
Verified by Experts
Topper's Solved these Questions
MBD MOST EXPECTED MODEL TEST PAPER
MBD -HARYANA BOARD|Exercise SHORT ANSWER TYPE QUESTIONS|7 VideosMBD MOST EXPECTED MODEL TEST PAPER
MBD -HARYANA BOARD|Exercise LONG ANSWER TYPE QUESTIONS|5 VideosMBD MOST EXPECTED MODEL TEST PAPER
MBD -HARYANA BOARD|Exercise OBJECTIVE TYPE QUESTIONS (Answer the following questions in one or two sentences)|8 VideosHALOALKANES AND HALOARENES
MBD -HARYANA BOARD|Exercise LONG ANSWER TYPE QUESTIONS|10 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
MBD -HARYANA BOARD|Exercise Long Answer Type Question|4 Videos
Similar Questions
Explore conceptually related problems
MBD -HARYANA BOARD-MBD MOST EXPECTED MODEL TEST PAPER-VERY SHORT ANSWER TYPE QUESTIONS
- Briefly explain the effects of temperature on the solubility of solids...
Text Solution
|
- What are crystalline solids ? Give two examples.
Text Solution
|
- Name any two tests to distinguish Alcohol from Phenol.
Text Solution
|
- Calculate the standard EMF of a cell involving cell reaction. Zn+2Ag...
Text Solution
|
- Define adsorption. Give one example.
Text Solution
|
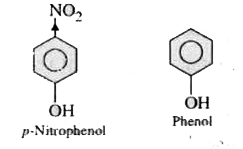
- p. Nitrophenol is more acidic than phenol. Explain why?
Text Solution
|
- Write the monomers used for preparing following polymers : (i) glypt...
Text Solution
|
- What are vitamins ? Name any two vitamins.
Text Solution
|
- What is carbylamines test for 1^(@) amine?
Text Solution
|
- Differentiate between minerals and ores.
Text Solution
|