Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PEARSON IIT JEE FOUNDATION-PERIODIC TABLE-LEVEL 3 APPLICATION-BASED QUESTIONS
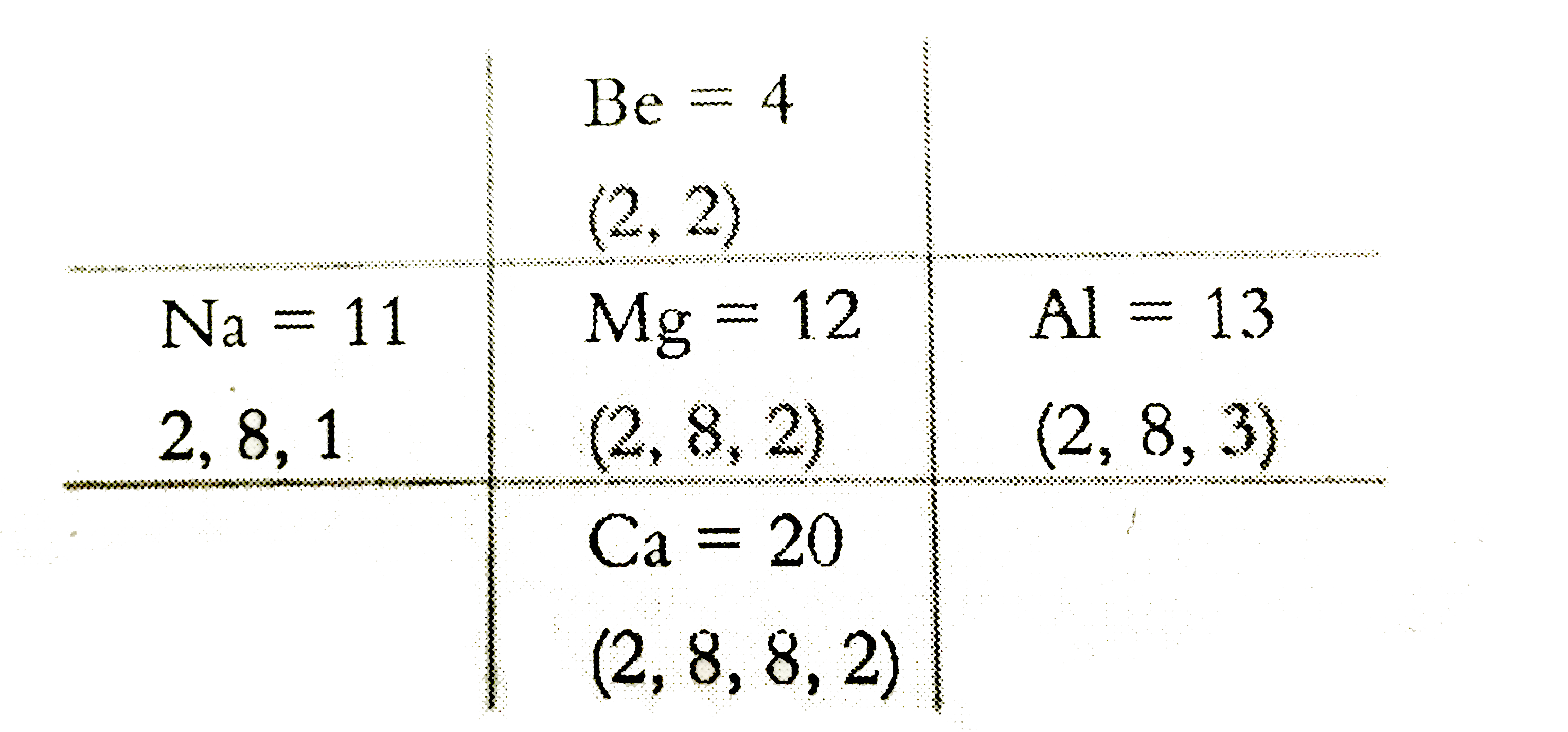
- In the modern periodic table, magnesium is surrounded by elements with...
Text Solution
|
- In the d-block elements as we move along a period the atomic radius ...
Text Solution
|
- Why are the values of electron affinity of the IIA group and IIB group...
Text Solution
|
- Why do some of the periodic properties like atomic size, electronega...
Text Solution
|
- Three elements X, Y and Z have atomic numbers 22, 40 and 72, respect...
Text Solution
|
- There is a sharp decrease in the ionisation energies from boron to a...
Text Solution
|