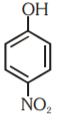
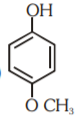
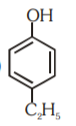
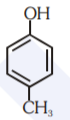
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NEET PREVIOUS YEAR (YEARWISE + CHAPTERWISE)-RE-NEET 2020-All Questions
- Which of the following is a free radical substitution reaction?
Text Solution
|
- The reaction of concentrated sulphuric acid with carbohydrates (C(12)H...
Text Solution
|
- Which of the following substituted phenols is the strongest acid?
Text Solution
|
- Match the compounds of Xe in column I with the molecular structure in ...
Text Solution
|
- The half life for a zero order reaction having 0.02M initial concentra...
Text Solution
|
- Identify the incorrect statement from the following:
Text Solution
|
- Match the following aspects with the respective metal.
Text Solution
|
- If 8g of a non-electrolyte solute is dissolved in 114g of n-octane to ...
Text Solution
|
- Match the coordination number and type of hybridisation with distribut...
Text Solution
|
- The number of angular nodes and radical nodes in 3s orbital are
Text Solution
|
- Identify the correct statement from the following
Text Solution
|
- Deficiency of which vitamin causes osteomalacia?
Text Solution
|
- Identify the wrongly matched pair.
Text Solution
|
- CH3CH2CH=CH2 overset(B2H6)underset(H2O,H2O2,OH^-)rarr Z What is Z?
Text Solution
|
- Identify the reaction from having top position in EMF series according...
Text Solution
|
- Match the element in column I with methods of purification in columb I...
Text Solution
|
- Which among the following salt solutions is basic in nature?
Text Solution
|
- In which of the sols, the colloidal particles are with negative charge...
Text Solution
|
- Which of the following acid will form an (a) Anhydride on heating and ...
Text Solution
|
- In a typical fuel cell, the reactants(R) and product (P) are:
Text Solution
|