Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
FULL MARKS-SAMPLE PAPER-8-PART-IV
- Derive an expression for electrostatic potential energy of the dipole ...
Text Solution
|
- Deduce the relation for the magnetic induction at a point due to an in...
Text Solution
|
- Obtain an expression for motional emf from Lorentz force.
Text Solution
|
- State Ampere's circuital law.
Text Solution
|
- The resolving power of a microscope is
Text Solution
|
- Derive an expression for De Broglie wavelength.
Text Solution
|
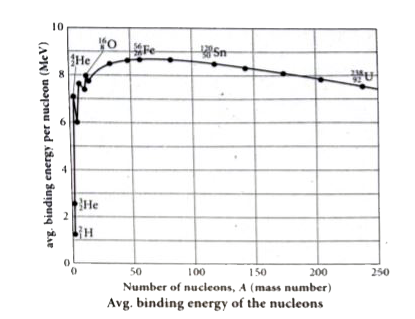
- Explain the variation of average binding energy with the mass number b...
Text Solution
|
- State Boolean laws.Elucidate how they are used to simplify Boolean exp...
Text Solution
|
- Modulation helps to reduce the antenna size in wireless communication-...
Text Solution
|
- What are the possible harmful effects of usage of Nanoparticles? Why? ...
Text Solution
|