(i) factors affecting solubility: There are three main factors which govem the solubility of solute they are: (i) Nature of the solute and solvent (ii) Temperature (iii) pressure
(i) Nature of the solute and solvent: the nature of the solute and solvent plays an important role in solubility. Although water dissolves an enormous variety of substances, both ionic and covalent, it does not dissolve everything . The phrase that scientists often use when predicting solubility is " like dissolves like". This expression means that dissolving occurs when similarities exist between the solvent and the solute. For example : Common salt is a polar compound and dissolves readily in polar sovent like water.
Non-polar compounds are soluble in non-polar solvents. For example, Fat dissolved in ether. But non-polar compounds, do not dissolve in polar solvents, polar compounds do not dissolve in non-polar solvents.
(ii) Effect of Temperature
Solubility of Solids in Liquid : Generally, solubility of a solid solute in a liquid solvent increases with increase in temperature. For example, a greater amount of sugar will dissolve in warm water than in cold water.
In endothermic process, solubility increases with increase in temperature.
In exothermic process, solubility decrease with increase in temperature.
Solubility of Gases in liquid : Solubility of gases in liquid decrease with increase in temperature. Generally, water contains dissolved oxygen. When water is boiled , the solubility of oxygen in water decreases , so oxygen escapes in the form of bubbles.
Aquatic animals live more in cold regions becuase, more amount of dissolved oxygen is present in the water of cold regions. This shows that hte solubility of oxygen in water is more at low temperatures.
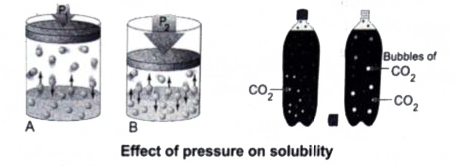
(iii) Effect of Pressure : Effect of pressure is observed only in the case of solubility of a gas in a liquid. When the pressure is increased, the solubility of a gas in liquid increases.
The common examples for solubility of gases in liquids are carbonated beverages, i.e., soft drinks, households cleanears containing aqueous solution of ammonia, formalinaqueous solution of formaldehyde etc.