Similar Questions
Explore conceptually related problems
Recommended Questions
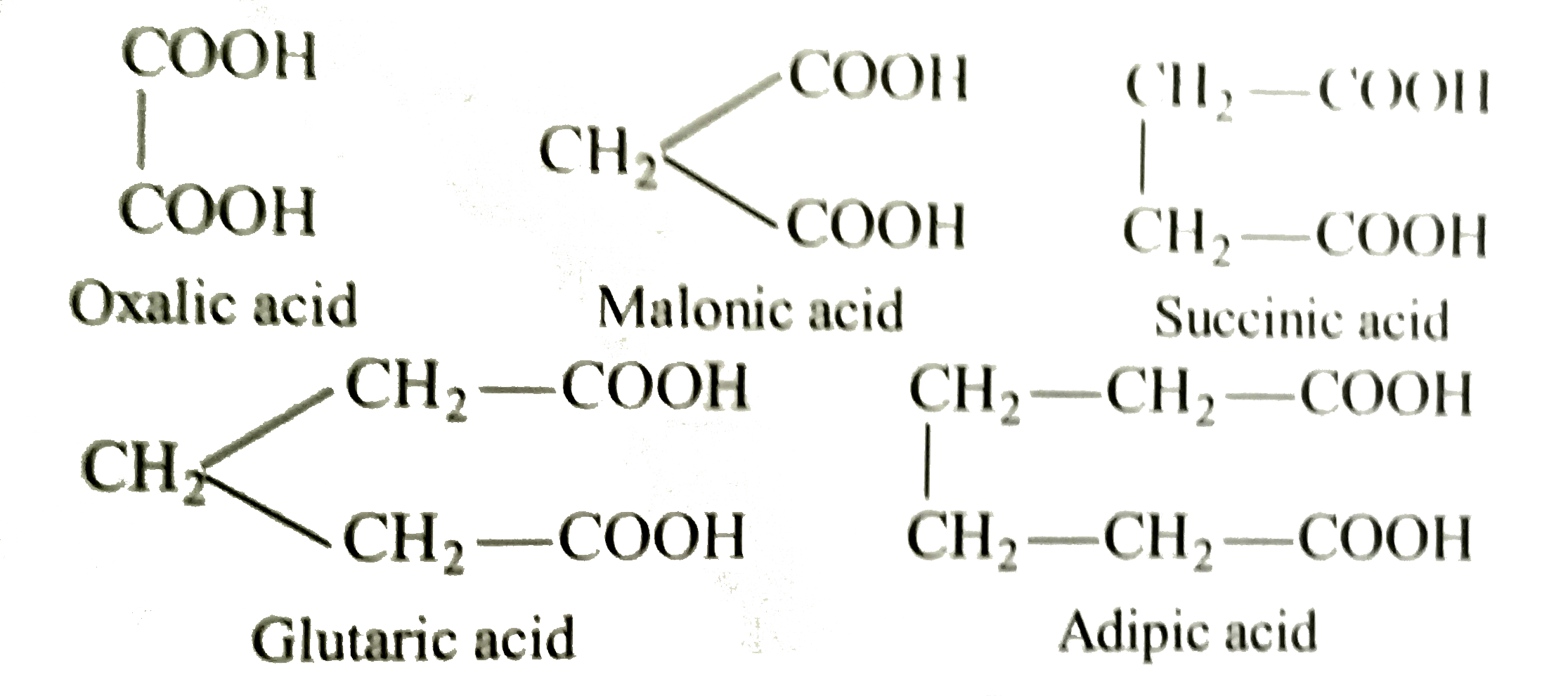
- Dicarboxylic acids have two carboxylic groups, e.g., Acidity of d...
Text Solution
|
- Dicarboxylic acids have carboxylic groups e.g. HOOC-COOH,oxalic acid...
Text Solution
|
- Dicarboxylic acids have carboxylic groups e.g. HOOC-COOH,oxalic acid...
Text Solution
|
- Dicarboxylic acids have carboxylic groups e.g. HOOC-COOH,oxalic acid...
Text Solution
|
- Which dicarboxylic acid on heating given monocarboxylic acid with two ...
Text Solution
|
- Dicarboxylic acids have two carboxylic groups, e.g., Acidity of d...
Text Solution
|
- Dicarboxylic acids have two carboxylic groups, e.g., Acidity of dicarb...
Text Solution
|
- Amino acids are classified as acidic, basic or neutral depending upon ...
Text Solution
|
- Amino acids are classified as acidic, basic or neutral depending upon ...
Text Solution
|