Similar Questions
Explore conceptually related problems
Recommended Questions
- A student is calculating the work during a reversible isothermal proce...
Text Solution
|
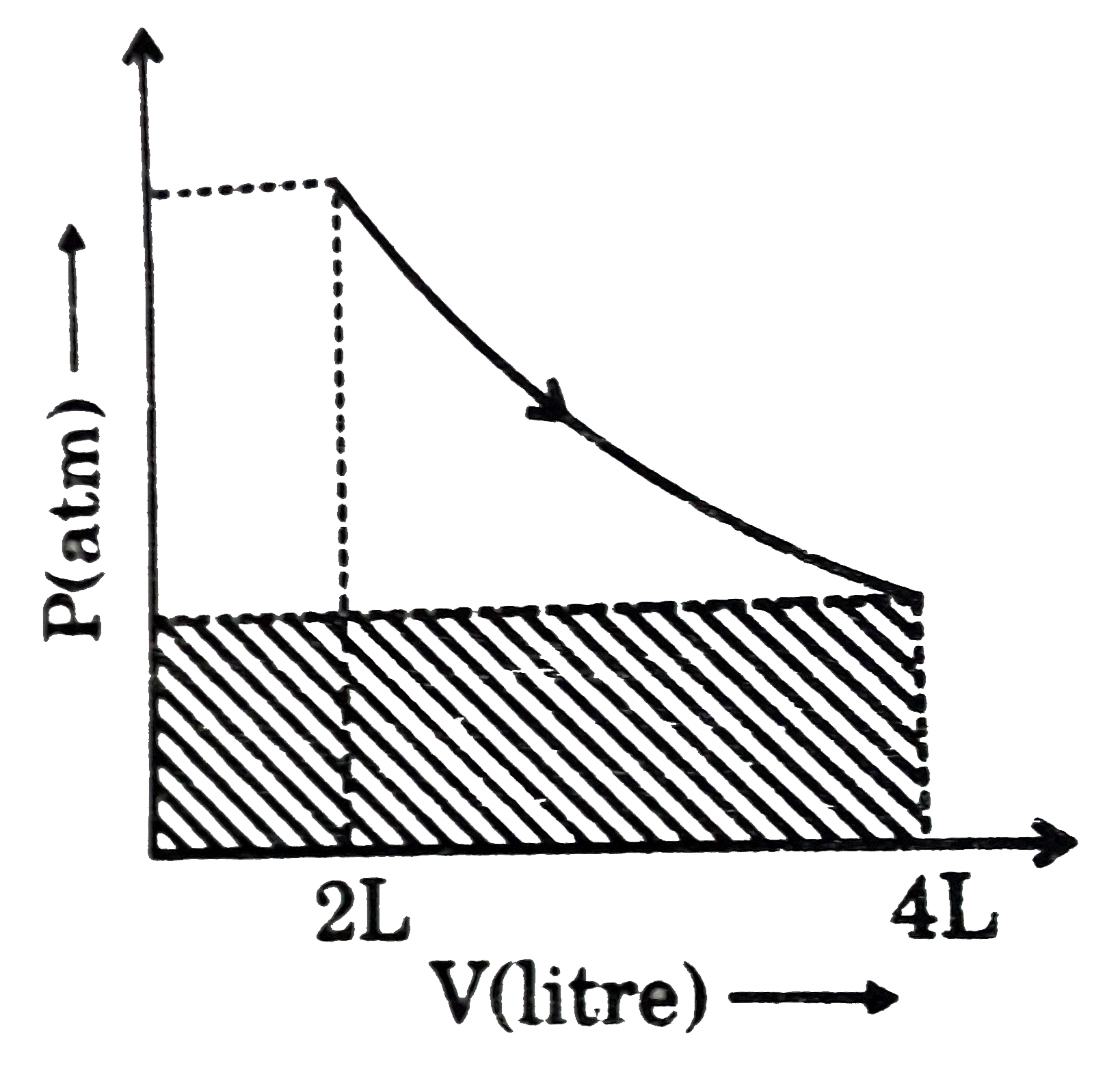
- One mole of an ideal gas is undergoing process as shown in figure then...
Text Solution
|
- A student is calculating the work during a reversible isothermal proce...
Text Solution
|
- Calculate DeltaG (in L atm) from the graph of an ideal gas undergoing ...
Text Solution
|
- A student is calculating the work during a reversible isothermal proce...
Text Solution
|
- If 1.5 L of an ideal gas at a apressure of 20 atm expands isothermally...
Text Solution
|
- Calculate the work done during isothermal reversible expansion of one ...
Text Solution
|
- Calculate the work done during isothermal reversible expansion expansi...
Text Solution
|
- 10 L आयतन और 1 atm दाब पर एक आदर्श गैस के 1 mol को एक अन्य 100 L वाले ...
Text Solution
|