Similar Questions
Explore conceptually related problems
Recommended Questions
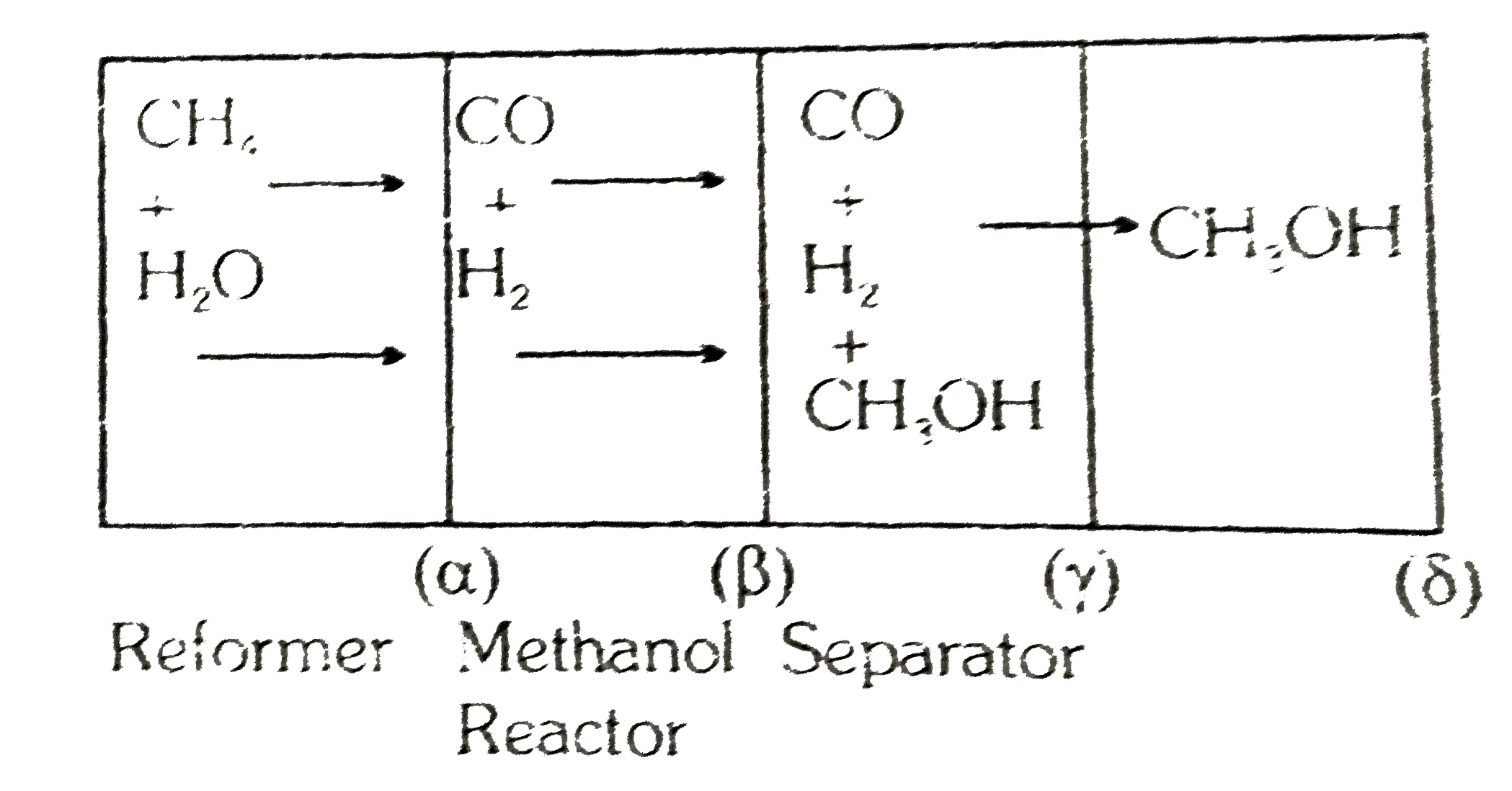
- Comprehension # 8 A factory, producing methanol, is based on the rea...
Text Solution
|
- What mass of CH(3)OH ("methanol") will be produced by reacting 1120L o...
Text Solution
|
- Comprehension # 8 A factory, producing methanol, is based on the react...
Text Solution
|
- Comprehension # 8 A factory, producing methanol, is based on the re...
Text Solution
|
- Comprehension # 8 A factory, producing methanol, is based on the react...
Text Solution
|
- A factory, producing methanol, is based on the reaction : CO+2H(2)ra...
Text Solution
|
- A factory, producing methanol, is based on the reaction : CO+2H(2)rarr...
Text Solution
|
- A factory, producing methanol, is based on the reaction : CO+2H(2)rarr...
Text Solution
|
- CH(3)-CH=CH(2)+Cl(2)+" Methanolic NaBr " to "product(s)" Which produ...
Text Solution
|