Similar Questions
Explore conceptually related problems
Recommended Questions
- Assertion (A): Furan is more soluble than DHF (Dihydrofuran, in H(2)...
Text Solution
|
- Assertion (A): NaOH is a stronger base than KOH. Reason (R ): KOH is...
Text Solution
|
- Assertion (A): The boiling point of diethyl ether is greater than fura...
Text Solution
|
- Assertion (A): Furan is more soluble than DHF (Dihydrofuran, in H(2)...
Text Solution
|
- Which solid is much more soluble in 1MHCI than in H(2)O ?
Text Solution
|
- Assertion (A) : H2S is more acidic than H2O. Reason (R) :H-S bond is...
Text Solution
|
- Statement-I: Fuan is more soluble than DHF Dihydrofuran, in H(2)O Stat...
Text Solution
|
- Assertion. Chlorine reacts more rapidly with H(2) than with D(2) . Rea...
Text Solution
|
- Assertion : H-bonding occurs in H(2)O due to larger size of O-atom. ...
Text Solution
|
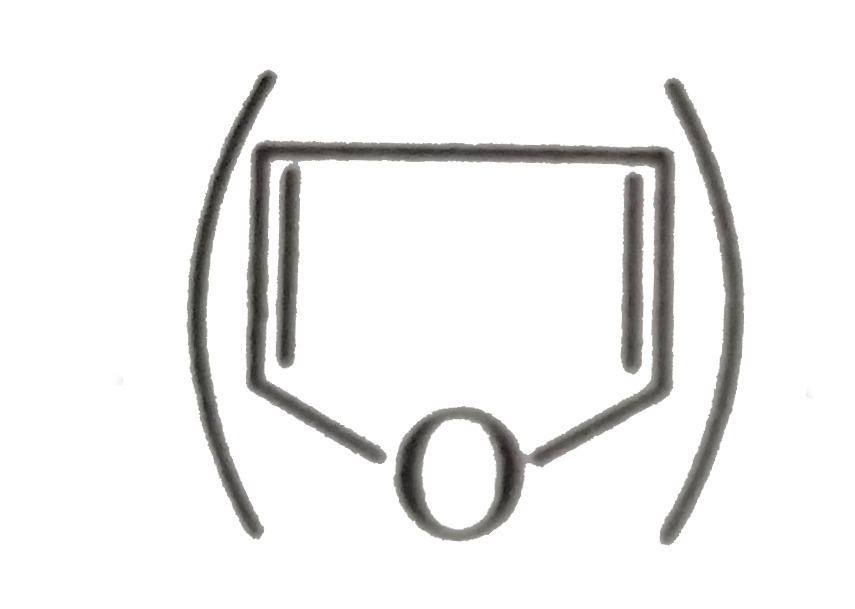 is more soluble than DHF (Dihydrofuran,
is more soluble than DHF (Dihydrofuran, 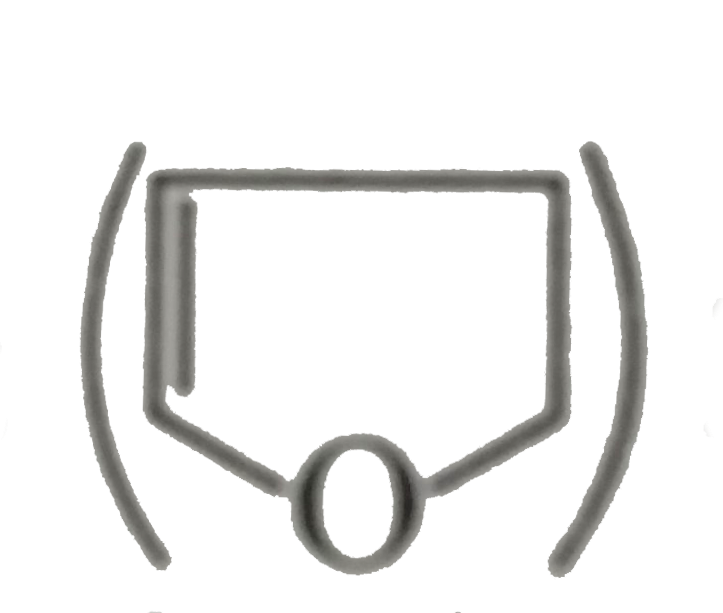 in `H_(2)O`.
in `H_(2)O`.