Similar Questions
Explore conceptually related problems
Recommended Questions
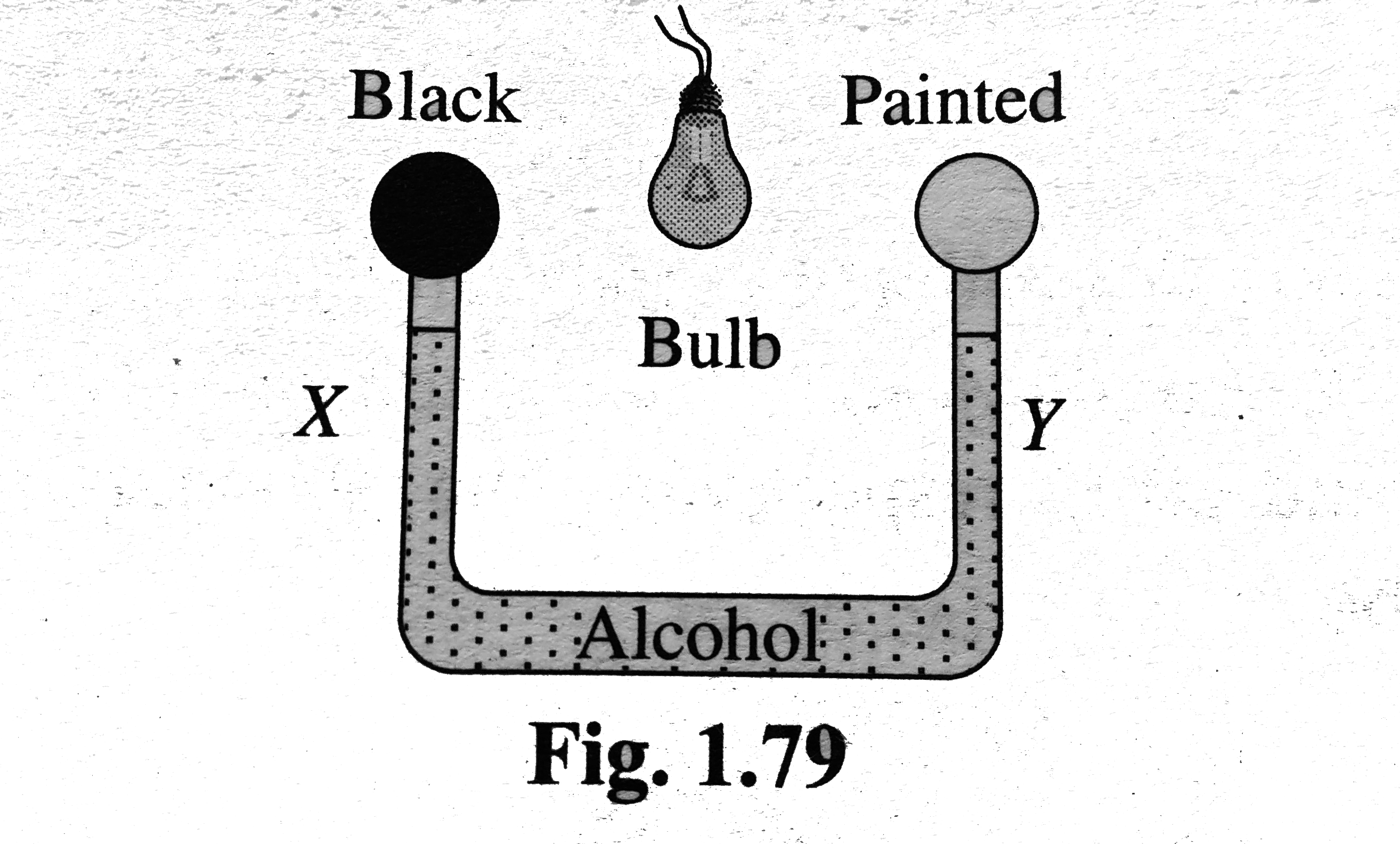
- Figure. Shows two air filled bulbs connected by a U-tube partly filled...
Text Solution
|
- Figure. Shows two air filled bulbs connected by a U-tube partly filled...
Text Solution
|
- Why is an electric light bulb not filled with air? Explain why argon o...
Text Solution
|
- Air is a homogeneous mixture of gases which also contains argon and ni...
Text Solution
|
- Air is a homogeneous mixture of gases which also contains argon and ni...
Text Solution
|
- Electric bulbs are filled with argon gas but not air. Explain.
Text Solution
|
- एल्कोहॉल से आंशिक रूप से भरी एक U-ट्यूब से बायु भरे दो बल्ब चित्रानुस...
Text Solution
|
- Two glass bulbs of volumes 500 c.c and 200 c.c are connected by a narr...
Text Solution
|
- Which gas is filled in electric bulbs/tubes:
Text Solution
|