Similar Questions
Explore conceptually related problems
Recommended Questions
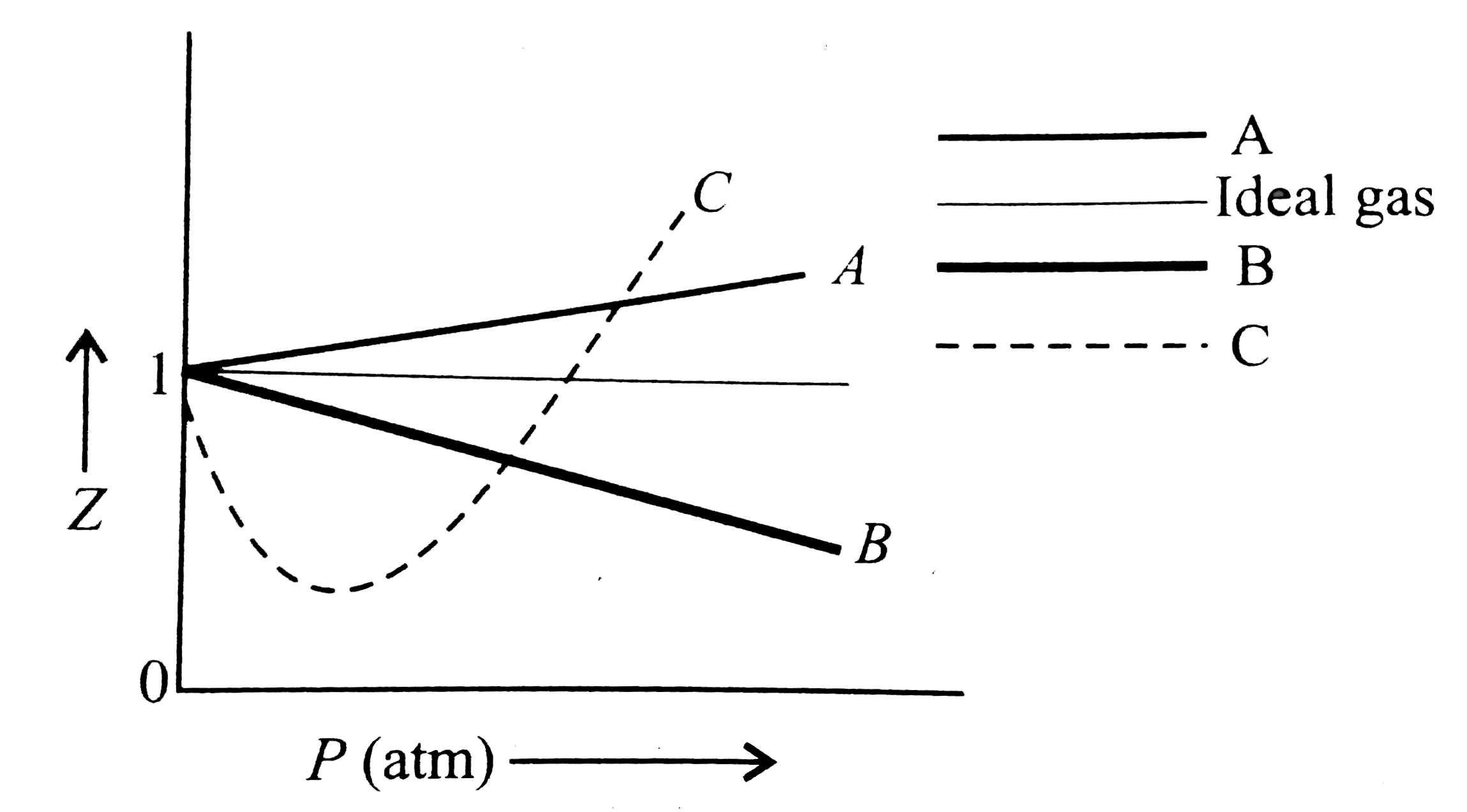
- The given graph represents the variations of compressibility factor Z=...
Text Solution
|
- The given graph represents the variations of compressibility factor Z=...
Text Solution
|
- For real gases, (PV)/(nRT) ........
Text Solution
|
- Compressibility factor of a gas is given by the equation Z=(PV)/(nRT)...
Text Solution
|
- The variation of compressibility factor (Z) with pressure (p in bar) ...
Text Solution
|
- The given graph represents the variations of compressibility factor Z=...
Text Solution
|
- Compressibility factor (Z=(PV)/(nRT)) is plotted against pressure ...
Text Solution
|
- Compressibility factor, Z of a gas is given as Z= PV"/"nRT For real ga...
Text Solution
|
- दिया गया आलेख (graph), तीन वास्तविक गैसों A, B तथा C के लिए संपीड़यता ग...
Text Solution
|