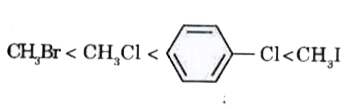
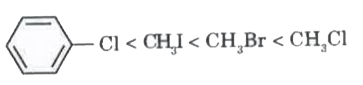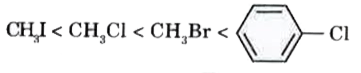A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
HALOALKANES AND HALOARENES
MODERN PUBLICATION|Exercise REVISION EXERCISE (Objective Questions) (Passage based questions)|10 VideosHALOALKANES AND HALOARENES
MODERN PUBLICATION|Exercise REVISION EXERCISE (Objective Questions) (Assertion Reason questions)|8 VideosHALOALKANES AND HALOARENES
MODERN PUBLICATION|Exercise MEMORY TEST (C. Choose the correct alternative)|12 VideosGENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
MODERN PUBLICATION|Exercise COMPETION FILE (Integer Type Numerical Value Type Questions)|5 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
MODERN PUBLICATION|Exercise UNIT PRACTICE TEST|5 Videos
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-HALOALKANES AND HALOARENES-REVISION EXERCISE (Objective Questions) (Multiple choice questions)
- Among the following, which one is chlorine containing insecticide?
Text Solution
|
- The boiling points of haloalkanes follow the order:
Text Solution
|
- Which of the following has highest dipole moment:
Text Solution
|
- Which of the following is not a polyhalogen compound?
Text Solution
|
- The ease of dehydrohalogenation of alkyl halide with alcoholic KOH is-
Text Solution
|
- Alkyl halides are prepared from alcohol by treating with:
Text Solution
|
- The organic chloro compound, which shows complete stereochemical inver...
Text Solution
|
- Which of the following reaction is most suitable for the preparation o...
Text Solution
|
- The reaction is:
Text Solution
|
- In the given alkyl halides which one has minimum boiling point?
Text Solution
|
- S(N)2 reaction will be fastest in:
Text Solution
|
- For the compounds, CH(3)Cl, CH(3)I, CH(3)Br and which of the followin...
Text Solution
|
- Which of the following organic compounds are formed by Wurtz reaction?
Text Solution
|
- In the preparation of alkyl halide from alcohol which of the following...
Text Solution
|
- Complete the following reaction
Text Solution
|
- Select the correct answer
Text Solution
|
- Select the correct answer
Text Solution
|
- Select the correct answer
Text Solution
|
- Select the correct answer
Text Solution
|
- Select the correct answer
Text Solution
|
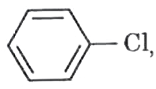 which of the following is the correct order of C-halogen bond length?
which of the following is the correct order of C-halogen bond length?