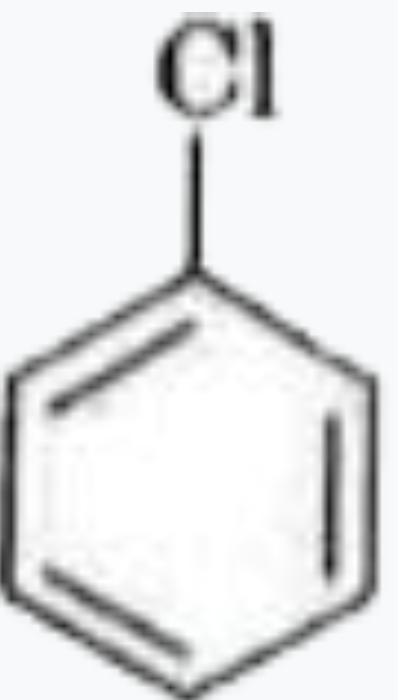
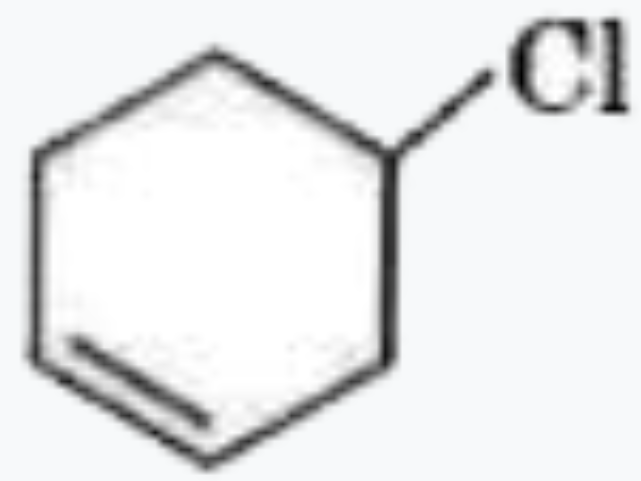
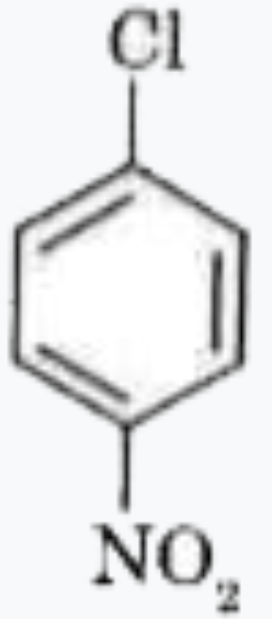
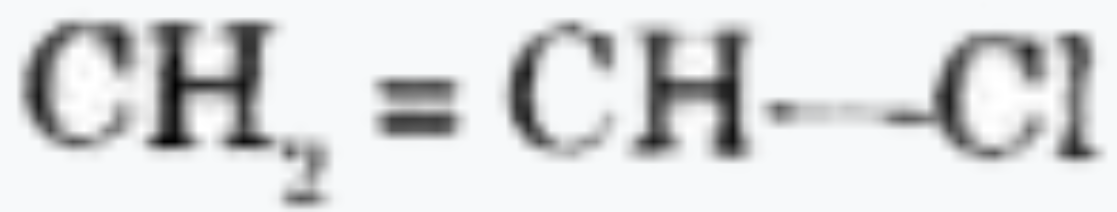A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
HALOALKANES AND HALOARENES
MODERN PUBLICATION|Exercise C. MULTIPLE CHOICE QUESTIONS (with more than one correct answers)|10 VideosHALOALKANES AND HALOARENES
MODERN PUBLICATION|Exercise D. MULTIPLE CHOICE QUESTIONS (based on the given passage / comprehension)|9 VideosHALOALKANES AND HALOARENES
MODERN PUBLICATION|Exercise A. MULTIPLE CHOICE QUESTIONS (with only one correct answer)|35 VideosGENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
MODERN PUBLICATION|Exercise COMPETION FILE (Integer Type Numerical Value Type Questions)|5 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
MODERN PUBLICATION|Exercise UNIT PRACTICE TEST|5 Videos
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-HALOALKANES AND HALOARENES-B. MULTIPLE CHOICE QUESTIONS (from competitive Examinations)
- The increasing order of reactivity of the following halides for the S(...
Text Solution
|
- 3-methyl-pent-2-ene on reaction with HBr in presence of peroxide forms...
Text Solution
|
- The major product obtained in the following reaction is:
Text Solution
|
- The major product of the following reaction is :
Text Solution
|
- Tertiary alkyl halides are practically inert to substitution by S(N)^(...
Text Solution
|
- The number of possible organobromine compounds which can be obtained i...
Text Solution
|
- Which of the following halides undergoes hydrolysis on warming with "w...
Text Solution
|
- The compound having longest C-Cl bond is
Text Solution
|
- The alkyl halides require to prepare by Wurtz reaction are
Text Solution
|
- The neopentyl halide in ethanol yields alkenes by E(1) mechanism due t...
Text Solution
|
- The major product of the following reaction is :
Text Solution
|
- Increasing order of reactivity of the following compounds for S(N)1 su...
Text Solution
|
- Heating of 2-chloro-1-phenylbutane with EtOK/EtOH gives X as the major...
Text Solution
|
- The major product of the following reaction is :
Text Solution
|
- The correct IUPAC name of the following compound is:
Text Solution
|
- The major product 'Y' in the following reaction is :
Text Solution
|
- The major product of the following reaction is :
Text Solution
|
- The correct statement(s) about the compound given below is (are):
Text Solution
|
- KI in acetone, undergoes S(N)2 reaction with each of P, Q, R and S. Th...
Text Solution
|
- In the following reaction, the major product is
Text Solution
|