Text Solution
Verified by Experts
Topper's Solved these Questions
ORGANIC COMPOUNDS CONTAINING NITROGEN
MODERN PUBLICATION|Exercise NCERT FILE MATCHING TYPE QUESTIONS|2 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
MODERN PUBLICATION|Exercise NCERT FILE ASSERTION AND REASON TYPE QUESTIONS|7 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
MODERN PUBLICATION|Exercise NCERT FILE MULTIPLE CHOICE QUESTION (TYPE -II)|10 VideosHALOALKANES AND HALOARENES
MODERN PUBLICATION|Exercise UNIT PRACTICE TEST|11 VideosPOLYMERS
MODERN PUBLICATION|Exercise Competition file (OBJECTIVE TYPE QUESTIONS) (C. MULTIPLE CHOICE QUESTIONS)(Integer Type Questions)|6 Videos
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-ORGANIC COMPOUNDS CONTAINING NITROGEN -NCERT FILE SHORT ANSWER TYPE QUESTIONS
- Explain why MeNH(2) is stronger base than MeOH ?
Text Solution
|
- What is the role of pyridine in the acelation reaction of amines?
Text Solution
|
- Under the reaction condition (acidic, basic) the coupling reaction of ...
Text Solution
|
- Predict the product of reaciton for aniline with bromine in non-polar...
Text Solution
|
- Arraange the following compounds in increasing order of dipole moment?...
Text Solution
|
- What is the structure and IUPAC name of the compound, allyl amine?
Text Solution
|
- Write down the IUPAC name of
Text Solution
|
- A compound Z with molecular formula C(3)H(9)N reacts with C(6)H(5)SO(2...
Text Solution
|
- A primary amine, RNH(2) can be reacted with CH(3)-X to get secondary a...
Text Solution
|
- Complete the following reaction.
Text Solution
|
- Why is aniline soluble in aqueous HCl?
Text Solution
|
- Suggest a route by which the following conversion can be accomplished.
Text Solution
|
- Identify A and B in the following reaction.
Text Solution
|
- How will you carry out the following conversion? (i) Toluene to p-to...
Text Solution
|
- Write following conversions (i) Nitrobenzene-Acetanilide (ii) Acet...
Text Solution
|
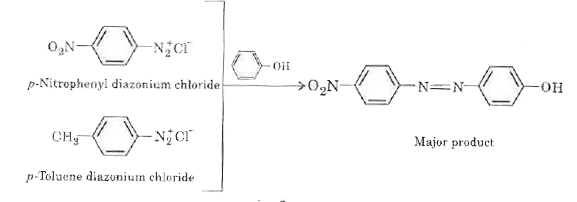
- A solution contains 1g mol. Each of p-toluene diazonium chloride and p...
Text Solution
|
- How will you bring out the following conversion?
Text Solution
|
- How will you carry out the following conversion?
Text Solution
|
- How will you carry out the following conversion?
Text Solution
|
- How will you carry out the following conversion?
Text Solution
|