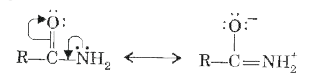
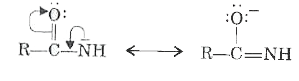
Text Solution
Verified by Experts
Topper's Solved these Questions
ORGANIC COMPOUNDS CONTAINING NITROGEN
MODERN PUBLICATION|Exercise Competition File (OBJECTIVE TYPE QUESTIONS) (MULTIPLE CHOICE QUESTIONS (M.C.Q)) (A)|30 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
MODERN PUBLICATION|Exercise Competition File (B) MULTIPLE CHOICE QUESTIONS|70 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
MODERN PUBLICATION|Exercise REVISION EXERCISES LONG ANSWER QUESTIONS|5 VideosHALOALKANES AND HALOARENES
MODERN PUBLICATION|Exercise UNIT PRACTICE TEST|11 VideosPOLYMERS
MODERN PUBLICATION|Exercise Competition file (OBJECTIVE TYPE QUESTIONS) (C. MULTIPLE CHOICE QUESTIONS)(Integer Type Questions)|6 Videos
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-ORGANIC COMPOUNDS CONTAINING NITROGEN -HIGHER ORDER THINKING SKILLS (ADVANCED LEVEL)
- tert-Butylamine cannot be prepared by action of ammonia on tert-butyl ...
Text Solution
|
- Explain : 2-aminoethanoic acid exists as a dipolar ion as does p-amino...
Text Solution
|
- Why are aryldiazonium ion more stable than alkyldiazonium ion?
Text Solution
|
- p-methoxyaniline is a stronger base than aniline but p-nitroaniline is...
Text Solution
|
- Can we prepare aniline by Gabriel phthalimide reaction?
Text Solution
|
- Sulphanilic acid is insoluble in water and organic solvents. Explain.
Text Solution
|
- Why is an amide more acidic than amine?
Text Solution
|
- Which is more basic PhNH(2) or Ph(2)NH ?
Text Solution
|
- An optically inactive compound (A) having molecular formula C(4)H...
Text Solution
|
- A colourless substance (A) is sparingly soluble in water and gives (B)...
Text Solution
|
- An organic compound A (C(3)H(5)N) on boiling with alkali gives ammonia...
Text Solution
|
- Identify (A) to (G) in the following reaction scheme:
Text Solution
|
- underset ((X)) underset ("Optically active") (C5 CH(13)N) underset (N2...
Text Solution
|