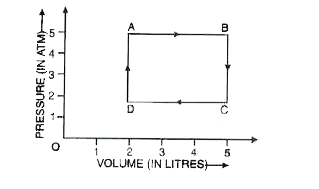
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
HEAT AND THERMODYNAMICS
MODERN PUBLICATION|Exercise Multiple Choice Questions (LEVEL-II)|55 VideosHEAT AND THERMODYNAMICS
MODERN PUBLICATION|Exercise Multiple Choice Questions (Level - III) (Questions From AIEEE/JEE Examination)|17 VideosGRAVITATION
MODERN PUBLICATION|Exercise Recent Competitive Questions|8 VideosLAWS OF MOTION
MODERN PUBLICATION|Exercise Revision test|43 Videos
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-HEAT AND THERMODYNAMICS-Recent Competitve Questions
- One mole of an ideal gas undergoes cyclic change as shown. The work do...
Text Solution
|
- The temperature of a gas contained in a closed vessel of constant volu...
Text Solution
|
- Hot water cools from 60^(@)C" to "50^(@)C in the first 10 min and to 4...
Text Solution
|
- The efficiency of Carnot's heat engine is 0.5 when the temperature of ...
Text Solution
|
- A perfect gas at 27^(@)C is heated at constant pressure so as to doubl...
Text Solution
|
- The quantities of heat required to raise the temperatures of two coppe...
Text Solution
|
- Which one of the following is v(m)-T graph for perfectly black body ? ...
Text Solution
|
- A hot body is allowed to cool. The surrounding temperature is constant...
Text Solution
|
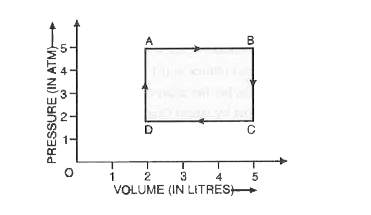
- One mole of an ideal gas is taken from A to B, from B to C and then ba...
Text Solution
|
- For which combination of working temperatures of source and sink the e...
Text Solution
|
- Two stars A and B radiate maximum energy at the wavelength of 360 nm a...
Text Solution
|
- What is the source temperature of the Carnot engine required to get 70...
Text Solution
|
- A cycle tyre bursts suddenly. What is the type of this process ?
Text Solution
|
- In anomalous expansion of water at what temperature, the density of wa...
Text Solution
|
- 1 gram of ice is mixed with 1 gram of steam. At themal equilibrium, th...
Text Solution
|
- Water is heated from 0^(@)C" to "10^(@)C, then its volume
Text Solution
|
- The average energy of molecules in a sample of oxygen gas at 300K are ...
Text Solution
|
- In an isochoric process
Text Solution
|