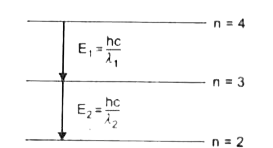
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ATOMS, MOLECULES AND NUCLEI
MODERN PUBLICATION|Exercise MCQ (Level -III)|9 VideosATOMS, MOLECULES AND NUCLEI
MODERN PUBLICATION|Exercise Recent Competitive Questions|40 VideosATOMS, MOLECULES AND NUCLEI
MODERN PUBLICATION|Exercise Recent Competitive Questions|40 Videos15 MAGNETIC EFFECTS OF CURRENTS
MODERN PUBLICATION|Exercise Recent Competitive Questions|31 VideosCURRENT ELECTRICITY
MODERN PUBLICATION|Exercise RECENT COMPETITIVE QUESTIONS|32 Videos
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-ATOMS, MOLECULES AND NUCLEI-MCQ (Level -II)
- The wavelength of the first spectral line in the Balmer series of hydr...
Text Solution
|
- The wavelength of the first line of Lyman series for hyrogen atom is e...
Text Solution
|
- Electron in hydrogen atom first jumps from third excited state to seco...
Text Solution
|
- Hydrogen atom is excited from ground state to another state with princ...
Text Solution
|
- A diatomic molecules is made of two masses m(2) and m(2) which are sep...
Text Solution
|
- Hydrogen atom from excited state comes to the ground state by emitting...
Text Solution
|
- The binding energies per nucleon for a deuteron and an alpha - particl...
Text Solution
|
- The transition from the state n =4 to n =3 is a hydrogen like atom res...
Text Solution
|
- The intensity of X-rays from a coolidge tube is plotted against wavele...
Text Solution
|
- An H2 atom and Li^(++) ion are both in the second excited state. If l(...
Text Solution
|
- In the nuclear fusion reaction ""(1)^(2)H+""(1)^(3)H to ""(2)^(4)He+n ...
Text Solution
|
- The binding energy of electron in a hydrogen atom is 13.6 eV, the ener...
Text Solution
|
- When a U^(238) nucleus originally at rest decays by emitting an alpha ...
Text Solution
|
- A radioactive sample at any instant has its disintegration rate 5000 d...
Text Solution
|
- The electric potential between a proton and an electron is given by V ...
Text Solution
|
- A nucleus disintegrates into two nuclear parts which have their veloci...
Text Solution
|
- The B.E. per nucleon of ""(1)H^(2) and ""(2)He^(4) are 1.1 MeV and 7 M...
Text Solution
|
- An alpha -particle of energy 5 MeV is scattered through 180^@ by a fix...
Text Solution
|
- The diagram shows the energy level of an electron in a certain atom. W...
Text Solution
|
- The intensity of gamma-"rays" from a given source is I. On passing thr...
Text Solution
|