A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SOME BASIC CONCEPTS OF CHEMISTRY
MODERN PUBLICATION|Exercise MULTIPLE CHOICE QUESTION (LEVEL-II)|100 VideosSOME BASIC CONCEPTS OF CHEMISTRY
MODERN PUBLICATION|Exercise MULTIPLE CHOICE QUESTION (LEVEL-III)|5 VideosSOLUTIONS
MODERN PUBLICATION|Exercise Recent Examination Questions|13 VideosSTATES OF MATTER
MODERN PUBLICATION|Exercise RECENT EXAMINATION QUESTIONS|12 Videos
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-SOME BASIC CONCEPTS OF CHEMISTRY -RECENT EXAMINATION QUESTION
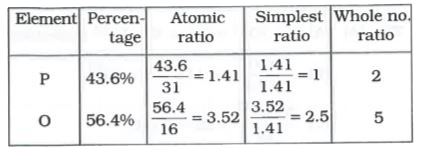
- A phosphorus oxide has 43.6% phosphorus (at. mass=31). The empirical ...
Text Solution
|
- 80 g of oxygen contains as many atoms as in
Text Solution
|
- Excess of carbon dioxide is passed through 50 mL of 0.5 M calcium hydr...
Text Solution
|
- 50 cm^3 of 0.2N HCl is titrated against 0.1N NaOH solution. The titr...
Text Solution
|
- A mixture of CaCl2 and NaCl weighing 4.44g is treated with sodium c...
Text Solution
|
- The equivalent mass of a certain bivalent metal is 20 . The molecular...
Text Solution
|
- The total number of electrons in 18 mL of water (density = 1 g mL^(-1)...
Text Solution
|
- The volume of 0.1 M oxalic acid that can be completely oxidized by 20 ...
Text Solution
|
- The number of water molecules present in a drop of water weighing 0.18...
Text Solution
|
- Empricial formula of a compound is CH(2)O and its molecular mass is 90...
Text Solution
|
- The mass of 112cm^(3) " of " NH(3) gas at STP is
Text Solution
|
- 10 g of a mixture of BaO and CaO requires 100cm^(3) of 2.5 M HCl to r...
Text Solution
|
- 25 cm^(3) of oxalic acid completely neutralised 0.064 g of sodium hydr...
Text Solution
|
- What amount of dioxygen (in gram) contains 1.8 xx 10^22 molecules ?
Text Solution
|
- 20 ml of acetic acid reacts with 20 ml of ethyl alcohol to form ethyl ...
Text Solution
|
- The mass of oxygen gas which occupies 5.6 litres at STP would be
Text Solution
|