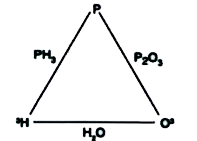
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SOME BASIC CONCEPTS OF CHEMISTRY
MODERN PUBLICATION|Exercise MULTIPLE CHOICE QUESTION (LEVEL-III)|5 VideosSOME BASIC CONCEPTS OF CHEMISTRY
MODERN PUBLICATION|Exercise RECENT EXAMINATION QUESTION|15 VideosSOME BASIC CONCEPTS OF CHEMISTRY
MODERN PUBLICATION|Exercise RECENT EXAMINATION QUESTION|15 VideosSOLUTIONS
MODERN PUBLICATION|Exercise Recent Examination Questions|13 VideosSTATES OF MATTER
MODERN PUBLICATION|Exercise RECENT EXAMINATION QUESTIONS|12 Videos
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-SOME BASIC CONCEPTS OF CHEMISTRY -MULTIPLE CHOICE QUESTION (LEVEL-II)
- How much time (in hours ) would it take to distribute one avogardo num...
Text Solution
|
- Arrange the following in the order of increasing mass (atomic mass: O ...
Text Solution
|
- Which one of the following sets of compounds correctly illustrate the...
Text Solution
|
- 20.0 kg of N2(g) and 3.0 kg of H2(g) are mixed to produce NH3(g) . The...
Text Solution
|
- Mole fraction of the solute in a 1.00 molal aqueous solution is
Text Solution
|
- What is the volume of CO2 liberated (in litres) at 1 atmosphere and...
Text Solution
|
- A 100% pure sample of a divalent metal carbonate weighing 2 g on compl...
Text Solution
|
- The equivalent mass of a certain bivalent metal is 20 . The molecular...
Text Solution
|
- The total number of electrons in 18 mL of water (density = 1 g mL^(-1)...
Text Solution
|
- Two solutions of HCI, A and B, have concentrations of 0.5 N and 0.1 M...
Text Solution
|
- Avogadro number (6.022 xx 10^23) of carbon atoms are present in
Text Solution
|
- The volume of 0.1 M Ca(OH)2 required to neutralize 10 mL of 0.1 N H...
Text Solution
|
- An aqueous solution of 6.3 g of oxalic acid dihydrate is made upto 250...
Text Solution
|
- How many moles of electrons weigh one kilogram?
Text Solution
|
- Mixture X = 0.02 mol of [Co (NH3)5 SO4] Br Brand 0.02 mol of [Co (N...
Text Solution
|
- Which has maximum number of atoms?
Text Solution
|
- Number of atoms in 588.6 g Fe (atomic mass of Fe = 55.86 g "mol"^(-1) ...
Text Solution
|
- What volume of hydrogen gas at 273K and 1 atm pressure will be consum...
Text Solution
|
- 6.02 xx 10^20 molecules of urea are present in 100 ml of its solutio...
Text Solution
|
- One mole of magnesium nitride on reaction with excess of water gives
Text Solution
|