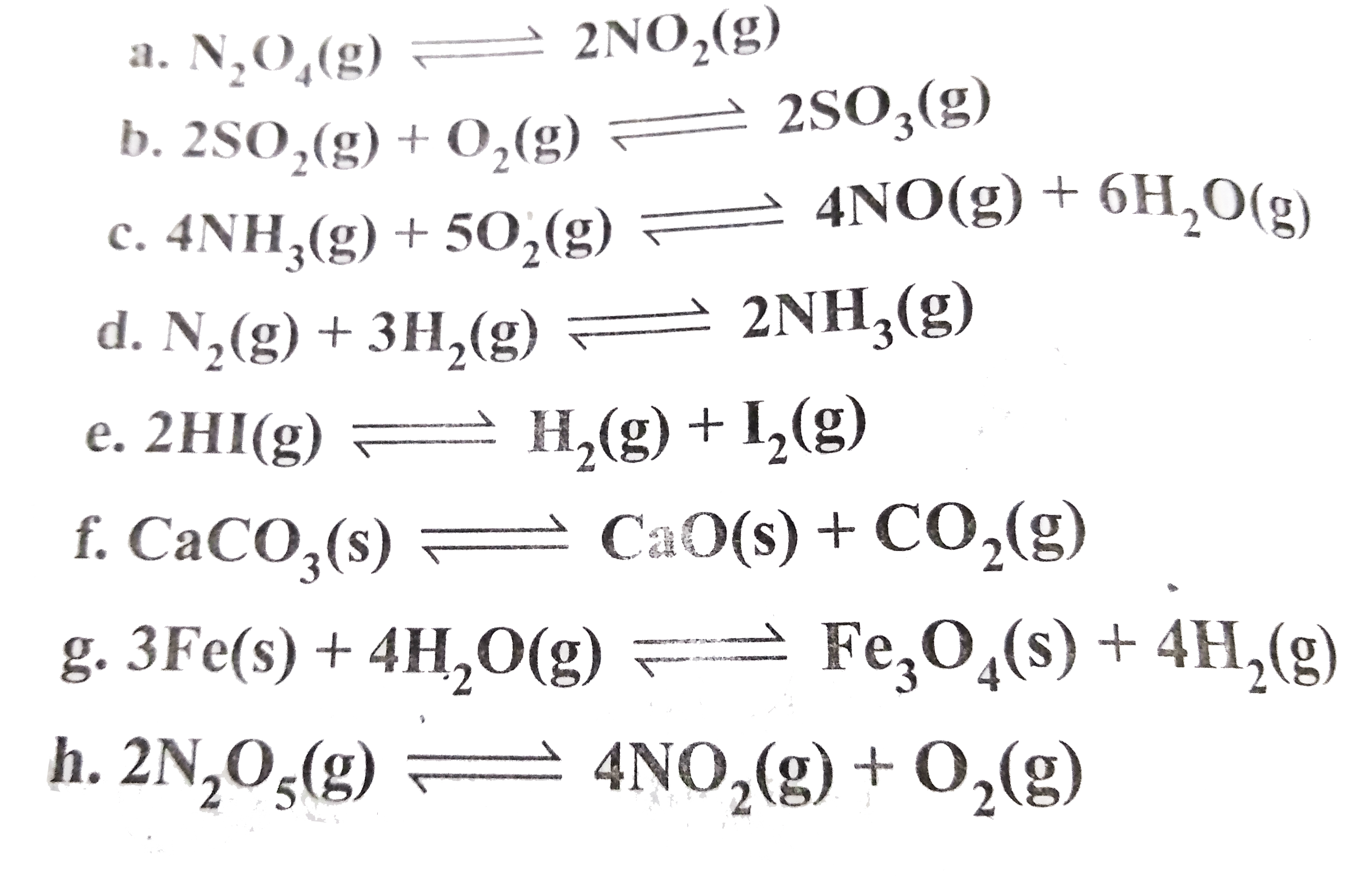Similar Questions
Explore conceptually related problems
Recommended Questions
- Write the expression for equilibrium constant for the following reacti...
Text Solution
|
- Write the expression for equilibrium constant for the following reacti...
Text Solution
|
- In the reaction, A + B hArr2C, at equilibrium, the concentration of A ...
Text Solution
|
- Write units of rate constant for zero order and for the second order r...
Text Solution
|
- The order of a reaction is 3/2. If concentration is expressed in mol.....
Text Solution
|
- For a reaction a, A to Products , the units of rate constant are ...
Text Solution
|
- For a reaction a, A to Products , the units of rate constant are ...
Text Solution
|
- Write units of rate constant for zero order and for the second order r...
Text Solution
|
- Write an expression for equilibrium constant with respect to concentra...
Text Solution
|