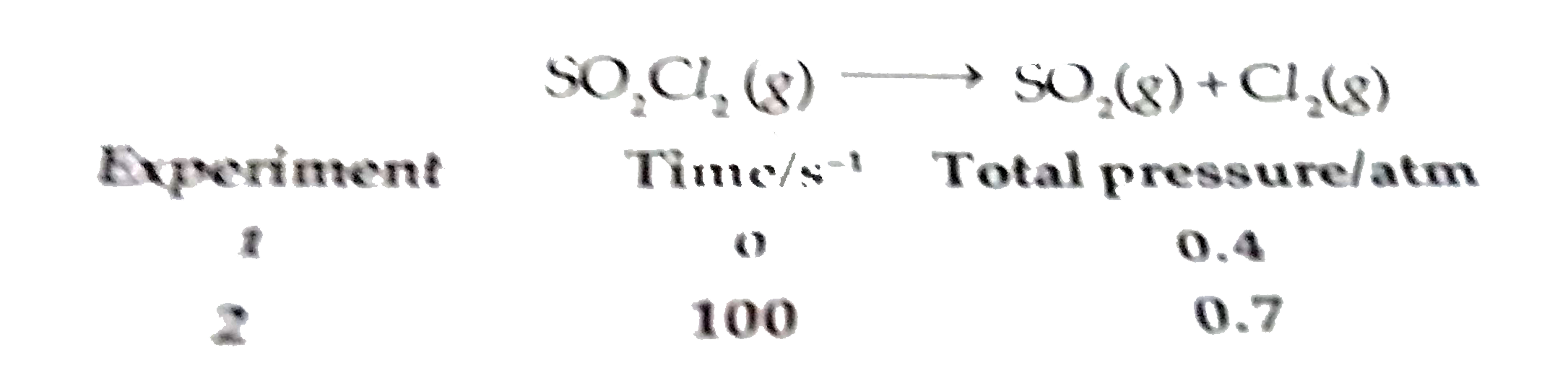Similar Questions
Explore conceptually related problems
Recommended Questions
- The following data were obtained during the first order thermal decomp...
Text Solution
|
- The following data were obtained during the first order thermal decomp...
Text Solution
|
- The following data were obtained during first order thermal decomposit...
Text Solution
|
- The following data were obtained during the first order decomposition ...
Text Solution
|
- The following data were obtained during the first order thermal decomp...
Text Solution
|
- The following data were obtained during the first order thermal decomp...
Text Solution
|
- The following data were obtained during the first order thermal decomp...
Text Solution
|
- स्थिर आयतन पर, SOCl(2) के प्रथम कोटि के ताप अपघटन पर निम्नांकित आँकड़े...
Text Solution
|
- The following data were obtained during the first order thermal decomp...
Text Solution
|