The molar conductance of an electrolytic solution decreases with increase in concentration. At low concentrations, the molar conductivity of strong electrolytes is found to obey, the following equation :
`^^_(m)=^^_(m)^(oo)-bsqrtC`
where b is a constant and `^^_(m)` is called the molar conductivity at infinite dilution. Thus, `^^_(m)=^^_(m)^(oo)` at infinite dilution, i.e., when `Cto0`
The variation of molar conductivity with concentration can be studied by plotting the values of `^^_(m)` against square root of concentration `(sqrtC)`. It has been observed that the variation of `^^_(m)` with concentration depends to a great extent on the type of electrolyte. In case of strong electrolytes, the change in `^^_(m)` with `(sqrtC)` is small. In case of strong electrolytes, the plots can be extrapolated to zero concentration. This gives the limiting value of molar conductance when the con- centration approaches zero and this value is called, molar conductance at infinite dilution. The plot of `^^_(m)` versus `(sqrtC)` for KCI solution is shown in Fig. It is denoted by `^^_(m)^(oo)`. However, in the case of weak electrolytes, we cannot obtain the molar conductance at infinite dilution `(^^_(m)^(oo))` by extrapolation of the `^^_(m)` versus `(sqrtC)` plots. The behaviour of `CH_(3)COOH` solution is also shown in figure.
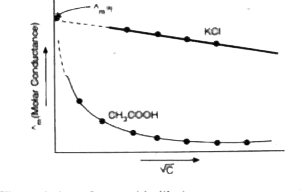
The variation of `^^_(m)` with dilution can be explained on the basis of number of ions in solution. The number of ions furnished by an electrolyte in solution depends upon the degree of dissociation with dilution. With the increase in dilution, the degree of dissociation increases and as a result molar conductance increases. (`^^_(m)^(oo)` corresponds to degree of dissociation equal to 1 i.e., the whole of the electrolyte dissociates.)
The degree of dissociation may be defined as
`alpha=(^^_(m)^(c))/(^^_(m)^(oo))`
Where `alpha` is the degree of dissociation, `^^_(m)` is the the molar conductance at concentration C and `^^_(m)^(oo)` is the molar conductance at infinite dilution.
However, in the case of strong electrolytes, there is no increase in the number of ions with dilution because strong electrolytes are completely ionised in solution at all concentrations (by definition). But there are strong forces of attraction between the ions of opposite charges called inter-ionic forces. Due to these inter-ionic forces, the conducting power of the ions is less in concentrated solutions. With dilution, the ions go far apart from one another and inter-ionic forces decreases. Therefore, molar conductance increases with dilution.
