Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MBD -HARYANA BOARD-ELECTROCHEMISTRY-LONG ANSWER TYPE QUESTIONS
- (i) Give three differences, between EMF and Potential difference. ...
Text Solution
|
- (i) Define specific and molar conductivity and give relation between t...
Text Solution
|
- What are fuel cells ? Discuss briefly hydrogen-oxygen fuel cell ?
Text Solution
|
- What is galvanic cell ? Discuss briefly with one example.
Text Solution
|
- Define an electrochemical cell. Discuss the working of Cu-Zn cell.
Text Solution
|
- How is molar conductivity related to concentration of the electrolyte ...
Text Solution
|
- How does conductivity of a solution varies with dilution ?
Text Solution
|
- How can we measure the single electrode potential ? Explain with one e...
Text Solution
|
- Describe the construction and working of Normal Hydrogen Electrode.
Text Solution
|
- Predict the products of electrolysis in each of the following: (i) A...
Text Solution
|
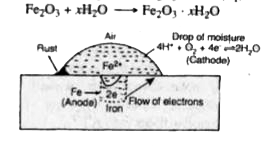
- Discuss the electrochemical theory of rusting.
Text Solution
|
- Calculate E("Cell")^(@)Cu//Cu^(2+)||Ag^(+)\\Ag E(Cu)^(@)=0.34V,E(Ag)...
Text Solution
|