Text Solution
Verified by Experts
Topper's Solved these Questions
GENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
MBD -HARYANA BOARD|Exercise LONG ANSWER TYPE QUESTIONS|3 VideosGENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
MBD -HARYANA BOARD|Exercise VERY SHORT ANSWER TYPE QUESTIONS|12 VideosELECTROCHEMISTRY
MBD -HARYANA BOARD|Exercise LONG ANSWER TYPE QUESTIONS|12 VideosHALOALKANES AND HALOARENES
MBD -HARYANA BOARD|Exercise LONG ANSWER TYPE QUESTIONS|10 Videos
Similar Questions
Explore conceptually related problems
MBD -HARYANA BOARD-GENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS -SHORT ANSWER TYPE QUESTIONS
- How is copper extracted from low gade ores or scraps ?
Text Solution
|
- The value of DeltafG^@ for formation of Cr2O3 is -540 kJ mol^(-1) and ...
Text Solution
|
- Why is copper matte put in silica lined converter ?
Text Solution
|
- Why is reduction of a metal oxide easier if the metal is formed in liq...
Text Solution
|
- What is hydrometallurgy? Explain with an example.
Text Solution
|
- (a) What is the role of cryolite in the metallurgy of aluminium ? (b...
Text Solution
|
- Why is the extraction of copper from pyrites more difficult than that ...
Text Solution
|
- What is the role of depressant in froth floatation process?
Text Solution
|
- Describe a method for refining nickel.
Text Solution
|
- Copper can be extracted by hydrometallurgy but not zinc. Explain.
Text Solution
|
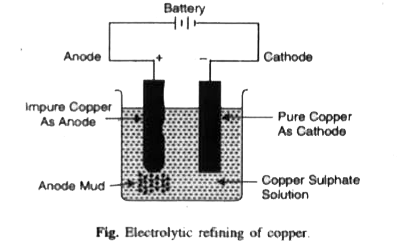
- Name the common elements present in the anode mud in electrolytic refi...
Text Solution
|
- How can you separate alumina from bauxite ore associated with silica ?...
Text Solution
|
- Giving examples, differentiate between roasting and calcination.
Text Solution
|
- Difine calcination with one example.
Text Solution
|
- How is cast iron different from pig iron?
Text Solution
|
- Why copper matte is put in silica lined converter ?
Text Solution
|
- The choice of a reducing agent in a particular case depends on thermod...
Text Solution
|
- Explain zone refining with a diagram
Text Solution
|
- Outline the principles of refining of metals by the following methods ...
Text Solution
|
- Outline the principle of refining of metals by (i) Zone refining. ...
Text Solution
|