Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MBD -HARYANA BOARD-P BLOCK ELEMENTS-LONG ANSWER TYPE QUESTION
- Describe Ostwald process for the manufacture of Nitric acid. Give uses...
Text Solution
|
- (i) Ammonia is good complexing agent. Explain. (ii) PCI exists as [...
Text Solution
|
- Name the various hydrides of group 16 elements. Arrange these hydrides...
Text Solution
|
- Write the names and formulae of any five oxyacids of sulphur.
Text Solution
|
- (a) What are interhalogen compounds ? Give examples. (b) Why are ...
Text Solution
|
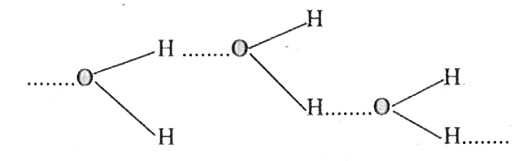
- Which of the two H2O or H2S has higher boiling point ? Explain.
Text Solution
|
- (a) Why does PCI3 fumes in moisture ? (b) NO2 dimerises to N2O4...
Text Solution
|
- Draw the structure of XeF2 and XeO3 . Write their shapes and hybridi...
Text Solution
|
- How are XeO3 and XeOF4 prepared ?
Text Solution
|
- Complete the following reaction (a) XeF2 + PCl5 to (b) NH4 NO...
Text Solution
|
- Explain the following: (b) Draw structure of IF^(-) . (c) Why a...
Text Solution
|
- Account for the following: (a) Molecular nitrogen N2 is not partic...
Text Solution
|
- Explain the following: (a) H2S is a gas while H2O is liquid at roo...
Text Solution
|
- List the important oxoacids of phosphorus and give their structures.
Text Solution
|
- Name five oxoacids of phosphorus and their formulae.
Text Solution
|
- (a) Arrange the following in the order of property indicated for each...
Text Solution
|
- (a) Draw the structure of following (i) XeF6 (i) XeOF4 Describe ...
Text Solution
|
- Define allotropy. Name the important allotropic forms of sulphur.
Text Solution
|
- (a) The basic character among the hydrides of group-15 elements decrea...
Text Solution
|
- (a) Draw the structure of XeF2 and BrF3 (b) Give the structures of ...
Text Solution
|