Text Solution
Verified by Experts
Topper's Solved these Questions
STATES OF MATTER : GASES AND LIQUIDS
MODERN PUBLICATION|Exercise Revision Exercises (Objective Questions)(Passage Based Questions)|10 VideosSTATES OF MATTER : GASES AND LIQUIDS
MODERN PUBLICATION|Exercise Revision Exercises (Objective Questions)(True or False Questions)|10 VideosSTATES OF MATTER : GASES AND LIQUIDS
MODERN PUBLICATION|Exercise NCERT FILE NCERT (EXemplar Problems) (Assertion and Reason Type Questions)|6 VideosSOME BASIC CONCEPTS OF CHEMISTRY
MODERN PUBLICATION|Exercise COMPETITION FILE (INTEGER TYPE AND NUMERICAL VALUE TYPE QUESTIONS)|10 VideosSTRUCTURE OF ATOM
MODERN PUBLICATION|Exercise Unit Practice Test|13 Videos
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-STATES OF MATTER : GASES AND LIQUIDS-NCERT FILE NCERT (EXemplar Problems) (Long Answer Questions)
- Isotherms of carbon dioxide at various temperature are represented in ...
Text Solution
|
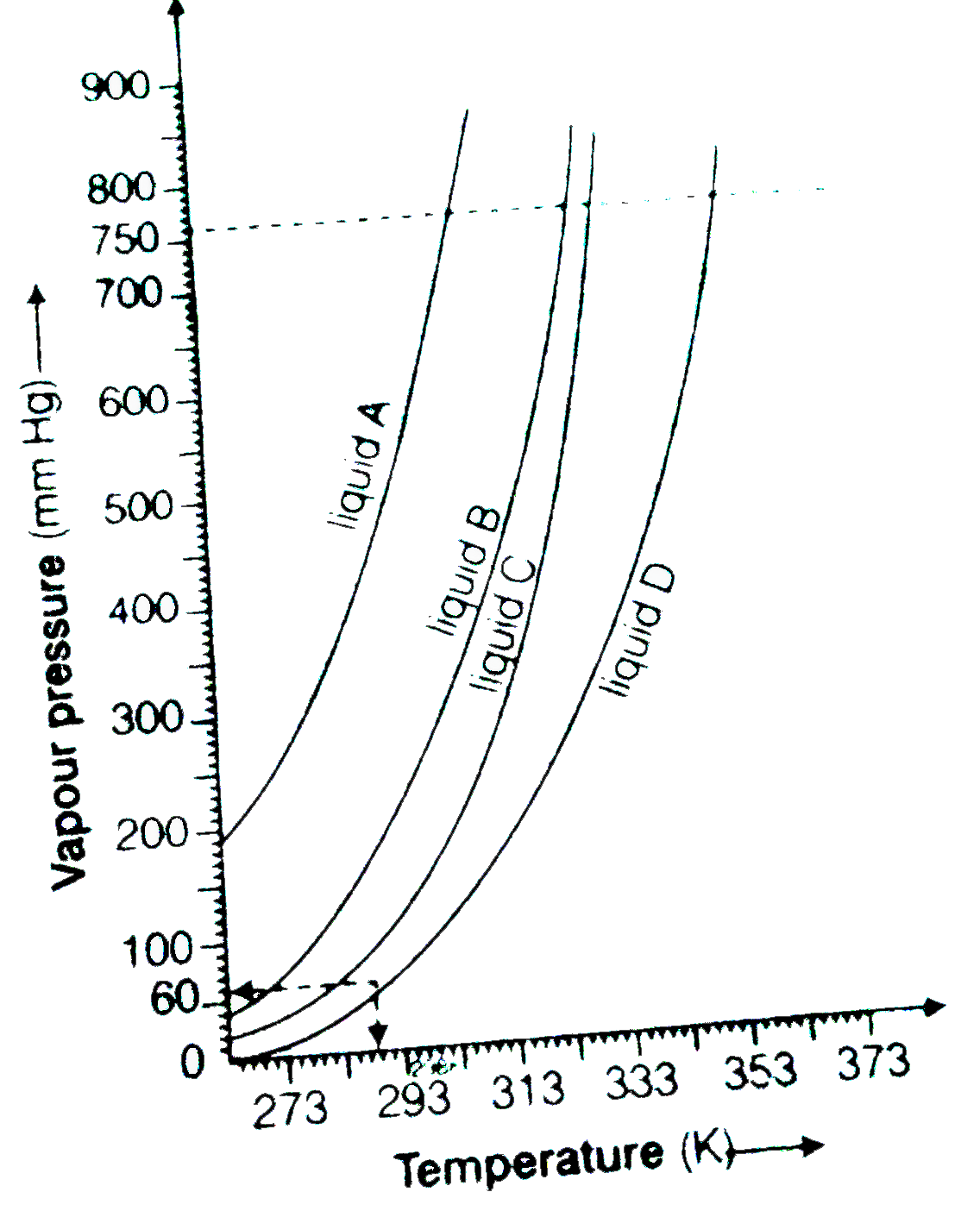
- The variation of vapour of different liquids with temperature is shown...
Text Solution
|
- Why does the boundary between liquid phase and gaseous phase disappear...
Text Solution
|
- Why does sharp glass edge become smooth on heating it upto its melting...
Text Solution
|
- Explain the term 'laminar flow'. Is the velocity of molecules same in ...
Text Solution
|
- The pressure and volume of gas are changed as shown in the P-V diagram...
Text Solution
|