Text Solution
Verified by Experts
Topper's Solved these Questions
STATES OF MATTER : GASES AND LIQUIDS
MODERN PUBLICATION|Exercise Revision Exercises (Objective Questions)(True or False Questions)|10 VideosSTATES OF MATTER : GASES AND LIQUIDS
MODERN PUBLICATION|Exercise Revision Exercises (Objective Questions)(Fill in the blanks Questions)|10 VideosSTATES OF MATTER : GASES AND LIQUIDS
MODERN PUBLICATION|Exercise NCERT FILE NCERT (EXemplar Problems) (Long Answer Questions)|6 VideosSOME BASIC CONCEPTS OF CHEMISTRY
MODERN PUBLICATION|Exercise COMPETITION FILE (INTEGER TYPE AND NUMERICAL VALUE TYPE QUESTIONS)|10 VideosSTRUCTURE OF ATOM
MODERN PUBLICATION|Exercise Unit Practice Test|13 Videos
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-STATES OF MATTER : GASES AND LIQUIDS-Revision Exercises (Objective Questions)(Passage Based Questions)
- Deviation of real gases from ideal behaviour can be studied by plots o...
Text Solution
|
- Deviation of real gases from ideal behaviour can be studied by plots o...
Text Solution
|
- Deviation of real gases from ideal behaviour can be studied by plots o...
Text Solution
|
- Deviation of real gases from ideal behaviour can be studied by plots o...
Text Solution
|
- Deviation of real gases from ideal behaviour can be studied by plots o...
Text Solution
|
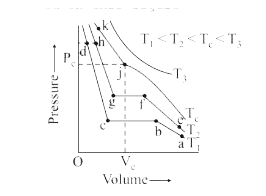
- Isotherms of carbon dioxide at various temperatures are represented in...
Text Solution
|
- Isotherms of carbon dioxide at various temperatures are represented in...
Text Solution
|
- Isotherms of carbon dioxide at various temperatures are represented in...
Text Solution
|
- The pressure and volume of gas are changed as shown in the P-V diagram...
Text Solution
|
- Assertion : Critical temperature of CO(2)" is "304 K, it cannot be liq...
Text Solution
|