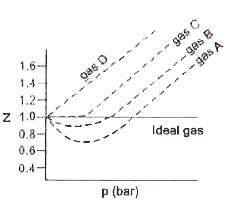
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
STATES OF MATTER : GASES AND LIQUIDS
MODERN PUBLICATION|Exercise COMPETITION FILE OBJECTIVE TYPE QUESTIONS (D. MULTIPLE CHOICE QUESTIONS)(Matrix Match Type Question)|2 VideosSTATES OF MATTER : GASES AND LIQUIDS
MODERN PUBLICATION|Exercise COMPETITION FILE OBJECTIVE TYPE QUESTIONS (D. MULTIPLE CHOICE QUESTIONS)(Integer Type Questions)|9 VideosSTATES OF MATTER : GASES AND LIQUIDS
MODERN PUBLICATION|Exercise COMPETITION FILE OBJECTIVE TYPE QUESTIONS (C. MULTIPLE CHOICE QUESTIONS)|10 VideosSOME BASIC CONCEPTS OF CHEMISTRY
MODERN PUBLICATION|Exercise COMPETITION FILE (INTEGER TYPE AND NUMERICAL VALUE TYPE QUESTIONS)|10 VideosSTRUCTURE OF ATOM
MODERN PUBLICATION|Exercise Unit Practice Test|13 Videos
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-STATES OF MATTER : GASES AND LIQUIDS-COMPETITION FILE OBJECTIVE TYPE QUESTIONS (D. MULTIPLE CHOICE QUESTIONS)
- The real gases show deviations from ideal gas behaviour. It is observe...
Text Solution
|
- Real gases do not follow the ideal gas equation perfectly under all co...
Text Solution
|
- Real gases do not follow the ideal gas equation perfectly under all co...
Text Solution
|
- The real gases show deviation from ideal gases donot follow Boyle's la...
Text Solution
|
- The real gases show deviations from ideal gas behaviour. It is observe...
Text Solution
|
- X and Y are two volatile liquids with molar weights of 10gmol^(-1) and...
Text Solution
|
- X and Y are two volatile liquids with molar weights of 10gmol^(-1) and...
Text Solution
|