Text Solution
Verified by Experts
Topper's Solved these Questions
S-BLOCK ELEMENTS ( ALKALI AND ALKALINE EARTH METALS )
MODERN PUBLICATION|Exercise NCERT FILE ( NCERT Exemplar Problems ( Multiple Choice Questions -I) )|21 VideosS-BLOCK ELEMENTS ( ALKALI AND ALKALINE EARTH METALS )
MODERN PUBLICATION|Exercise NCERT FILE ( NCERT Exemplar Problems ( Multiple Choice Questions -II) )|7 VideosS-BLOCK ELEMENTS ( ALKALI AND ALKALINE EARTH METALS )
MODERN PUBLICATION|Exercise Conceptual Question 2|10 VideosREDOX REACTIONS
MODERN PUBLICATION|Exercise COMPETITION FILE (Objective Questions) (C. MULTIPLE CHOICE QUESTIONS)|9 VideosSOME BASIC CONCEPTS OF CHEMISTRY
MODERN PUBLICATION|Exercise COMPETITION FILE (INTEGER TYPE AND NUMERICAL VALUE TYPE QUESTIONS)|10 Videos
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-S-BLOCK ELEMENTS ( ALKALI AND ALKALINE EARTH METALS ) -NCERT FILE ( NCERT Textbook Exercises)
- Potassium carbonate can be obtained by Solvay's process.
Text Solution
|
- Why is Li(2)CO(3) decomposed at a lower temperature whereas Na(2)CO(3)...
Text Solution
|
- Compare the solubility and thermal stability of the following compound...
Text Solution
|
- Starting from sodium chloride, how will you proceed to prepare (i) sod...
Text Solution
|
- What happens when (a) magensium in burnt in air, (b) quicklime is heat...
Text Solution
|
- Describe two important uses of each of the following: (a) casutic so...
Text Solution
|
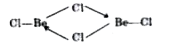
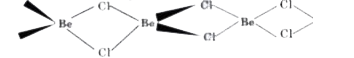
- Draw the structure of (i) BeCl2 (vapour) and (ii) BeCl2 (solid).
Text Solution
|
- The hydroxides and carbonates of sodium and potassium are easily solub...
Text Solution
|
- Describe the importance of the following: (a) limestone, (b) cement an...
Text Solution
|
- Why are lithium salts commonly hydrated while those of other alkali me...
Text Solution
|
- Why it LiF almost insoluble in water while LiCl is soluble not only in...
Text Solution
|
- Explain the significance of sodium, potassium, magnesium and calcium o...
Text Solution
|
- What happens when a. Sodium metal is dropped in water? b. Sodium m...
Text Solution
|
- Comment on each of the following observation: a. The mobilities of t...
Text Solution
|
- State as to why (a) a solution of Na(2)CO(3) is alkaline ? (b) alk...
Text Solution
|
- Write balanced quations for reaction between (a) Na2O2 and water (...
Text Solution
|
- How would you explain the following observations? (i) BeO is almost ...
Text Solution
|
- .Which of the alkali metal is having least melting point?
Text Solution
|
- Which one of the following alkali metals gives hydrated salts ?
Text Solution
|
- Which one of the alkaline earth metal carbonates is thermally the most...
Text Solution
|