Similar Questions
Explore conceptually related problems
Recommended Questions
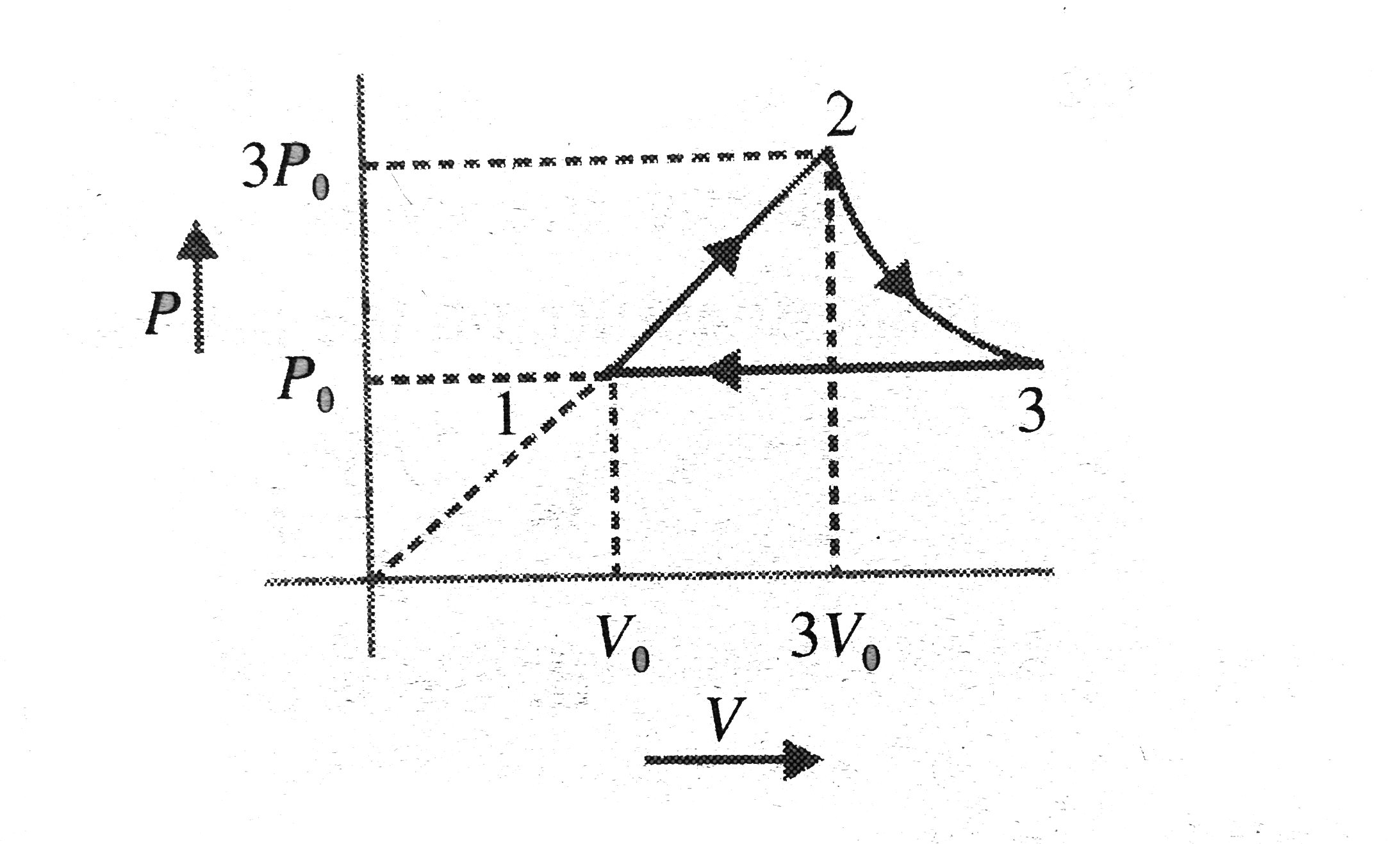
- A system undergoes three quasi-static process sequentially as indicate...
Text Solution
|
- If Pm stands for m{P{m}} , then prove that: 1+1. P1+2. P2+3. P3++ndotP...
Text Solution
|
- Let P(m) stand for nP(m). Then the expression 1.P(1)+2.P(2)+3.P(3)+......
Text Solution
|
- A thermodynamic process is shown in the following figure. The process ...
Text Solution
|
- Determine the work done by an ideal gas doing 1 rarr 4 rarr 3rarr 2 ra...
Text Solution
|
- A thermodynamic process is shown in Fig. The pressures and volumes cor...
Text Solution
|
- A thermodynamic process is shown in Fig. The pressures and volumes cor...
Text Solution
|
- A system undergoes three quasi-static process sequentially as indicate...
Text Solution
|
- An ideal gasa undergoes through folowing cyclic procees. 1-2 : Reversi...
Text Solution
|