Similar Questions
Explore conceptually related problems
Recommended Questions
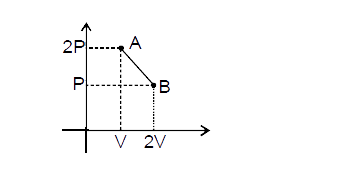
- The process AB is shown in the diagram. As the gas is taken from A to ...
Text Solution
|
- In a cyclic process shown in the figure an ideal gas is adial gas is a...
Text Solution
|
- In a cyclic process, a gas is taken from state A and B via path -I as ...
Text Solution
|
- An ideal gas is taken from point A to the point B, as shown in the P-V...
Text Solution
|
- A sample of ideal gas is taken through the cyclic process shown in the...
Text Solution
|
- Two moles of an ideal monoatomic gas is taken through a cycle ABCA as ...
Text Solution
|
- The process AB is shown in the diagram. As the gas is taken from A to ...
Text Solution
|
- n mol The ideal gas is shown in the figure AB Was taken through the pr...
Text Solution
|
- In a cyclic process shown in the figure on ideal gas is adiabatically ...
Text Solution
|