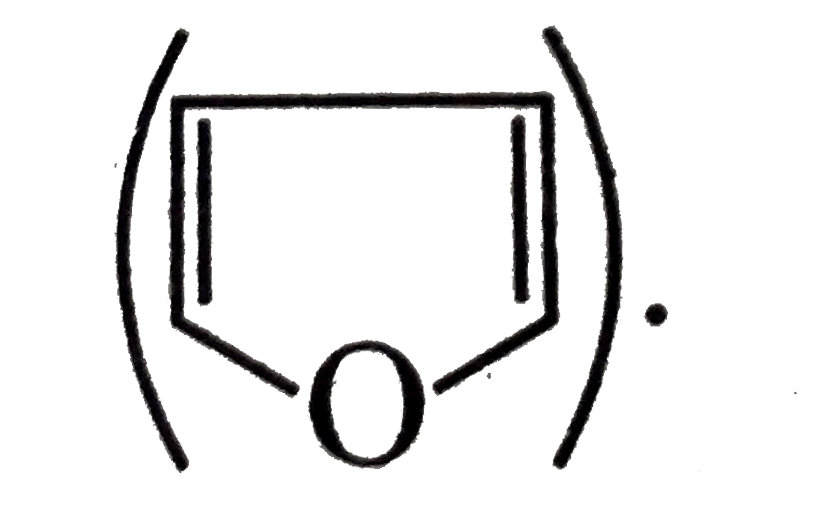
Similar Questions
Explore conceptually related problems
Recommended Questions
- Assertion (A): The boiling point of diethyl ether is greater than fura...
Text Solution
|
- Assertion (A): The boiling point of diethyl ether is greater than fura...
Text Solution
|
- Assertion (A): Furan is more soluble than DHF (Dihydrofuran, in H(2)...
Text Solution
|
- Butan-1-ol has higher boiling point than diethyl ether. Assign reason.
Text Solution
|
- Statement-I: The boiling pint of diethyl ehter is greater than furan S...
Text Solution
|
- Ethanol has higher boiling point diethyl ether or ethyl amine. Why ?
Text Solution
|
- Furan is a?
Text Solution
|
- Furan is a carbocyclic compound.
Text Solution
|
- Classify Furan according to the organic compound.
Text Solution
|