A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
HEAT AND TEMPERATURE
FIITJEE|Exercise COMPREHENSION TYPE|5 VideosHEAT AND TEMPERATURE
FIITJEE|Exercise ASSERTION REASON TYPE|1 VideosHEAT AND TEMPERATURE
FIITJEE|Exercise SOLVED PROBLEMS SUBJECTIVE|18 VideosGRAVITATION
FIITJEE|Exercise Numerical based Question|2 VideosKINEMATICS
FIITJEE|Exercise NUMERICAL BASED QUESTIONS DECIMAL TYPE|5 Videos
Similar Questions
Explore conceptually related problems
FIITJEE-HEAT AND TEMPERATURE-SOLVED PROBLEMS OBJECTIVE
- Two identical containers A and B with frictionless pistons contain the...
Text Solution
|
- What should be the length of steel and copper rods at 0^(@)C that the ...
Text Solution
|
- The average translational energy and the rms speed of molecules in a s...
Text Solution
|
- Two cylinders A and B fitted with pistons contain equal amounts of an ...
Text Solution
|
- P.V plotsfortwo gases during adiabatic processses are shown in the fig...
Text Solution
|
- What will be the efficiency of cycle as shown in the figure. 1to2...
Text Solution
|
- In a 10 m deep lake, the bottom is at a constant temperature of 4^(@)C...
Text Solution
|
- The intensity of the solar radiation is maximum for wavelength lamda(m...
Text Solution
|
- A body with an initial temperature theta(1) is allowed to cool in a su...
Text Solution
|
- A black body is at a temperature of 2880 K. The energy of radiation em...
Text Solution
|
- In a room where the temperature is 30^(@)C, a body cools from 61^(@)C ...
Text Solution
|
- Let barv,v(rms) and vp respectively denote the mean speed. Root mean s...
Text Solution
|
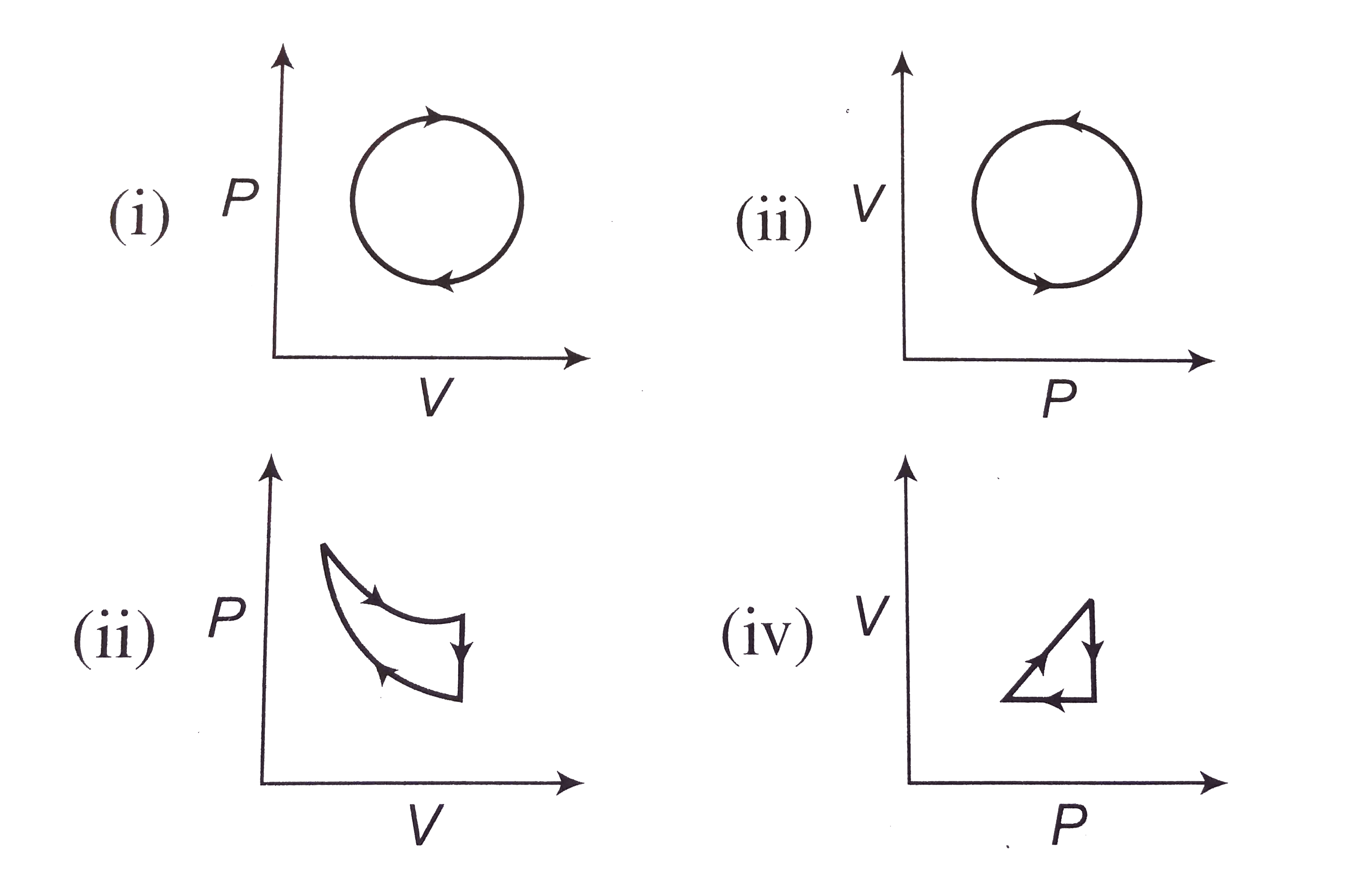
- The following are the P-V diagrams for cyclic processes for a gas. In ...
Text Solution
|
- A spherical black body of radius r radiates power P, and its rate of c...
Text Solution
|
- The solar constant for the earth is sum. The surface temperature of th...
Text Solution
|