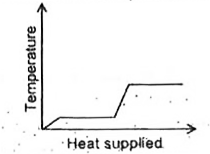
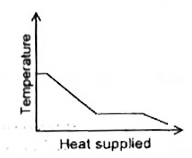
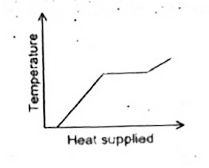
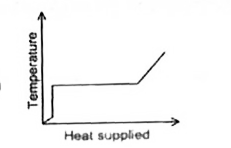
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
HEAT AND TEMPERATURE
FIITJEE|Exercise ASSIGNMENT PROBLEMS (OBJECTIVE) (LEVEL-I) (ASSERTION AND REASON)|2 VideosHEAT AND TEMPERATURE
FIITJEE|Exercise ASSIGNMENT PROBLEMS (OBJECTIVE) (LEVEL II)|20 VideosHEAT AND TEMPERATURE
FIITJEE|Exercise ASSIGNMENT PROBLEMS (SUBJECTIVE) (LEVEL II AND III)|15 VideosGRAVITATION
FIITJEE|Exercise Numerical based Question|2 VideosKINEMATICS
FIITJEE|Exercise NUMERICAL BASED QUESTIONS DECIMAL TYPE|5 Videos
Similar Questions
Explore conceptually related problems
FIITJEE-HEAT AND TEMPERATURE-ASSIGNMENT PROBLEMS (OBJECTIVE) (LEVEL-I)
- A liquid with coefficient of real volume expansion (gamma) is filled i...
Text Solution
|
- At room temperature (27^(@)C) the 'rms' speed of the molecules of a ce...
Text Solution
|
- Two moles of a monatomic gas is mixed with three moles of a diatomic g...
Text Solution
|
- A diatomic gas does 80 J of work when expanded isobarically. The heat ...
Text Solution
|
- A block of ice -10^(@)C is slowly heated and converted to steam at 100...
Text Solution
|
- When an ideal diatomic gas is heated at constant pressure, the fractio...
Text Solution
|
- If the density of a gas at NTP is 1.3mg//cm^(3) and velocity of sound ...
Text Solution
|
- The velocities of three molecules are 3v, 4v and 5v. Calculate their r...
Text Solution
|
- If the average kinetic energy of gas molecule at 27^(@)C is 6.21xx10^(...
Text Solution
|
- A substance of mass M kg requires a power input of P wants to remain i...
Text Solution
|
- The coefficient of linear expansion of a rod is alpha and its length i...
Text Solution
|
- Two idential container joined by a small pipe initially contain the sa...
Text Solution
|
- A cyclic process ABCD is shown in the figure P-V diagram. Which of the...
Text Solution
|
- A spherical black body with radius 12 cm radiates 640 w power at 500 K...
Text Solution
|
- m grams of a gas of molecules weight M is flowing in an insulated tub...
Text Solution
|
- Two metallic spheres S1 and S2 are made of the same material and have ...
Text Solution
|
- Two perfect gases at absolute temperature T(1) and T(2) are mixed. The...
Text Solution
|
- A pendulum clock keeps correct time at 0^(@)C. Its mean coefficient of...
Text Solution
|
- Two straight metallic strips each of thickness t and length l are rive...
Text Solution
|
- The radius of a ring is R and its coefficient of linear expansion is a...
Text Solution
|