Similar Questions
Explore conceptually related problems
Recommended Questions
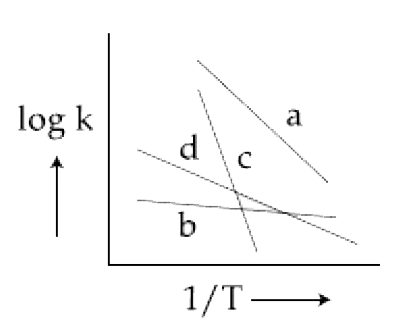
- Consider the following plots of rate constant versus (1)/(T) f...
Text Solution
|
- In a second order reaction, the plot of 1//(a-x) versus t is a straigh...
Text Solution
|
- For a reaction, consider the plot of In K versus 1//T given in the fig...
Text Solution
|
- Consider the following plots of rate constant versus (1)/(T) f...
Text Solution
|
- When a plot of log k versus 1//T of chemical reaction is made, the ene...
Text Solution
|
- The rate constant of a first order reaction follows the equation log...
Text Solution
|
- Consider the following plots of rate constant versus (1)/(T) for four ...
Text Solution
|
- Consider the following plots of rate constant versus (1)/(T) f...
Text Solution
|
- Rate constant k of a reaction varies with temperature according to the...
Text Solution
|